-
Thu16May2024Sun19May2024
-
Mon10Jun2024
-
Tue11Jun2024
-
Fri14Jun2024
-
Fri30Aug2024Mon02Sep2024
-
Thu26Sep2024Sun29Sep2024
-
Sun06Oct2024Tue08Oct2024
Latest News
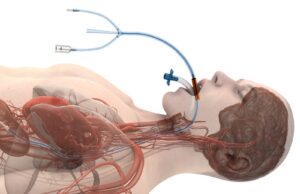
EVERCOOL AF study of oesophageal cooling system enrols its first patient
Heart Rhythm Clinical and Research Solutions (HRCRS) and the Real World Evidence (RWE) Consortium have enrolled the first patient in the EVERCOOL AF study,...
Features
Cardiac Rhythm News’ top stories of 2023
Which stories captured the attention of the electrophysiology community across 2023? Read our summary of the trending stories from across the Cardiac Rhythm News...
“Not just inventing the next stent”: The future of medtech relies...
Ian Meredith is a well-known figure within the field of interventional cardiology, not only for a distinguished academic and clinical career but also his...
Videos
Data from the PROMET and RELEASE studies provide important new insights into the safety and efficacy of rotational transvenous lead extraction (TLE), Christoph Starck (Berlin, Germany) and Peter Paul Delnoy (Zwolle, The Netherlands) tell Cardiac Rhythm News at the 2022 European...