 Abbott has today announced receipt of the CE mark for the TactiFlex ablation catheter, sensor enabled (SE), alongside US Food and Drug Administration (FDA) approval for an expanded indication of its FlexAbility ablation catheter, sensor enabled (SE).
Abbott has today announced receipt of the CE mark for the TactiFlex ablation catheter, sensor enabled (SE), alongside US Food and Drug Administration (FDA) approval for an expanded indication of its FlexAbility ablation catheter, sensor enabled (SE).
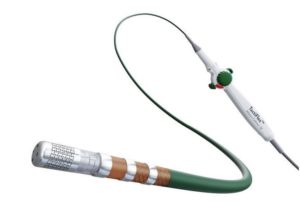
In a press release, the company describes TactiFlex, SE as the world’s first ablation catheter designed with a flexible tip and contact force sensing, intended to reduce procedure times and patients’ exposure to radiation compared to standard power ablation.
“When we treat complex ablation cases for people battling arrhythmias, we want to eliminate the arrhythmia and get our patients back to living their lives,” said Isabel Deisenhofer (German Heart Centre Munich, Munich, Germany). “The TactiFlex catheter’s data around using high-power during ablation will be game-changing for patients. When you combine these tools with Abbott’s EnSite X EP System, the innovation is truly opening new doors in patient care.”
Abbott’s TactiFlex catheter uses a tip design with a laser-cut pattern that flexes when in contact with the heart wall to direct irrigation flow to the treated tissue and to increase catheter stability by up to two-times for consistent therapy delivery.
The catheter generated strong clinical outcomes in the TactiFlex AF IDE study for its treatment using high-power ablation (between 40 and 50 Watts). The study showed the catheter created fast, safe lesions to treat the patient’s arrhythmia the first time with over 99% acute procedural success.
Abbott’s TactiFlex catheter is now available in Europe, Africa, Japan and Australia. It is currently undergoing FDA review for premarket approval.
FlexAbility, SE is intended to treat ventricular tachycardia (VT) in patients with non-ischemic cardiomyopathy (NICM). Abbott’s LESS-VT study was the first FDA-approved premarket trial to study the safety and effectiveness of ablation for the treatment of VT with NICM origin. Once treated with the FlexAbility catheter, SE, 80% of study patients were free from VT for at least six months post-procedure9. The data also showed statistically significant improvements in patients’ mental and physical quality-of-life measures.












