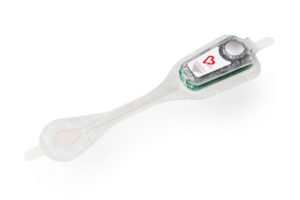
It was announced recently that Bardy Diagnostics (BardyDx) has entered into a distribution agreement with JNC Medical, a distributor of medical technologies based in Ottawa, Canada. The agreement grants JNC Medical the right to distribute the BardyDx Carnation Ambulatory Monitor (CAM), in the Canadian market.
“The collaboration with JNC Medical strengthens BardyDx’s international distribution and business development initiatives, which are key priorities for the Company this year,” said Ken Nelson, BardyDx chief commercial officer. “Combining BardyDx’s innovation and leadership in ambulatory cardiac monitoring with JNC Medical’s Canadian market-specific expertise, distribution logistics, and deep connections with Canadian physicians will accelerate penetration of the CAM patch into the global market.”
According to a press release, the partnership aims to enable access to the CAM patch for all Canadian patients and accelerate the transition away from legacy Holter technologies and outdated ECG reporting solutions. As part of its distribution strategy, JNC Medical intends to leverage its healthcare industry networks and physician demands for extended monitoring, encouraging a transition to digital monitoring technologies.
“We are excited to partner with BardyDx by assisting in the commercialisation of the market-disrupting CAM patch in Canada,” said JNC Medical business partner Christopher Mulvanny. “It is our goal to bring the world’s most innovative device technologies to Canadian physicians and patients, and the CAM patch is a perfect match to add to our growing portfolio.”
In preparation, BardyDx recently received a Health Canada Medical Device License, allowing the CAM patch to be marketed in Canada. Additionally, to support international expansion initiatives and as a compulsory requirement for entering the Canadian market, BardyDx was recently awarded MDSAP (Medical Device Single Audit Program) certification, a program involving a single regulatory audit of a medical device manufacturer’s quality management system that satisfies the requirements of multiple regulatory jurisdictions worldwide.












