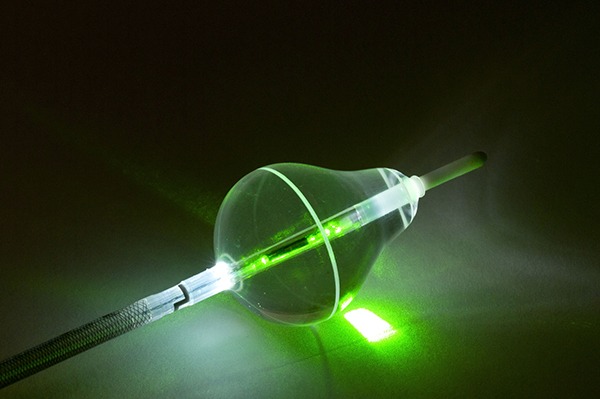
CardioFocus’ HeartLight Excalibur Balloon has been granted CE mark. The technology is designed for the treatment of atrial fibrillation.
The Excalibur balloon builds on the universal balloon design of the company’s HeartLight endoscopic ablation system. It is intended to optimise the speed and magnitude of target tissue contact during pulmonary vein isolation (PVI) procedures.
Heartlight endoscopic ablation system
The original HeartLight system comprises a visually-guided laser balloon technology. It is designed to treat patients whose heart arrhythmias are insufficiently controlled with medication. The system received approval from the Japanese Ministry of Health, Labour and Welfare earlier this year.
Supported by a distribution partnership with Japan Lifeline, HeartLight is due for commercial release in the first quarter of 2018. Approved by the US Food and Drug Administration (FDA), the device has been commercially available in the US since September 2016.
European Excalibur evaluation
In January of this year, CardioFocus announced an initial clinical evaluation of the Excalibur balloon, which sought to confirm the product’s design objectives.
“We are delighted by the results we experienced during our clinical evaluation of the HeartLight Excalibur Balloon,” says Petr Neužil from Na Homolce Hospital, in Prague, Czech Republic. “We consistently noted that the Excalibur Balloon is easier and faster to use, establishes significantly more contact with the vein and can consistently obtain an impressive antral position.”
In addition to a more compliant construction designed to enable adaptive vein conformance, the device incorporates Dynamic Response technology. This feature makes the balloon responsive to a range of techniques and pressure, while optimising vein contact. According to a company release, this will maximise the engagement of the balloon with the pulmonary veins. In addition, this should decrease the time required to complete ablation procedures.
The company plans to begin a controlled launch of Excalibur in Europe, beginning in the fourth quarter of this year.











