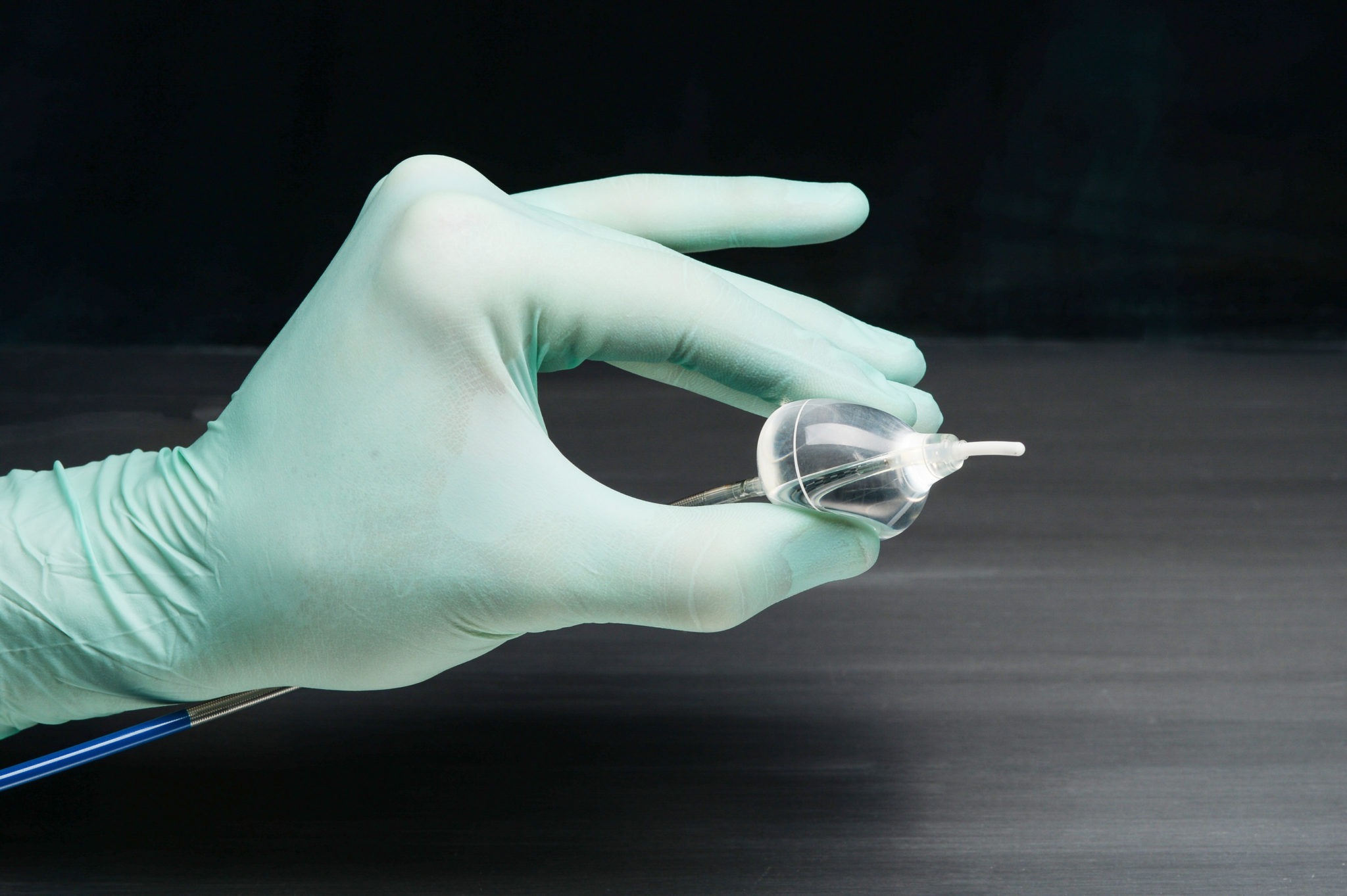
CardioFocus has announced that the first patients have been treated commercially with the recently-approved HeartLight X3 endoscopic ablation system in the USA. The cardiac ablation technology is designed to treat drug refractory, symptomatic paroxysmal atrial fibrillation (AF), the most common heart rhythm disorder.
Henry D Huang of Rush University Medical Center, Chicago, USA and David Kenigsberg Westside Regional Medical Center in Plantation, USA performed the first procedures using the X3 system.
“We are dedicated to providing our patients the most efficacious and innovative treatment options for AFib, a common but treatable heart condition,” Huang said. “As the initial medical centre in the USA to offer the HeartLight X3 system, we look forward to being able to provide effective symptom relief for Chicago-area patients safely, confidently and quicker than ever before. We are excited by the results we have seen thus far, which build upon the positive outcomes we experienced in treating more than 100 patients with previous versions of HeartLight.”
More than 2.3 million people in the USA suffer from AF, and the numbers are climbing along with the aging population, CardioFocus said. By 2050, AF is expected to affect 15.9 million people.
“Early on, I recognized the potential of the HeartLight system and after using it to treat more than 40 patients, I have been eager to offer the next-generation, HeartLight X3, to our patients here in South Florida,” said Kenigsberg. “I believe the technology has true game-changing potential in how it facilitates pulmonary vein isolation (PVI).”
The HeartLight X3 system received US Food and Drug Administration (FDA) approval for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation (PAF) in May. Approval was based on a comprehensive submission, including outcomes from the study of 60 HeartLight X3 patients over 12 months. All study endpoints were achieved, and the device achieved very rapid PVI, in as few as three minutes for a single pulmonary vein.
“Following the impressive clinical trial results of HeartLight X3, we have been equally encouraged by these initial real-world patient experiences,” said Burke T Barrett, chief executive officer at CardioFocus. “Following the swift FDA approval, we are pleased to have begun a focused commercial rollout of the HeartLight X3 system in the USA that will showcase its impressive array of features.”












