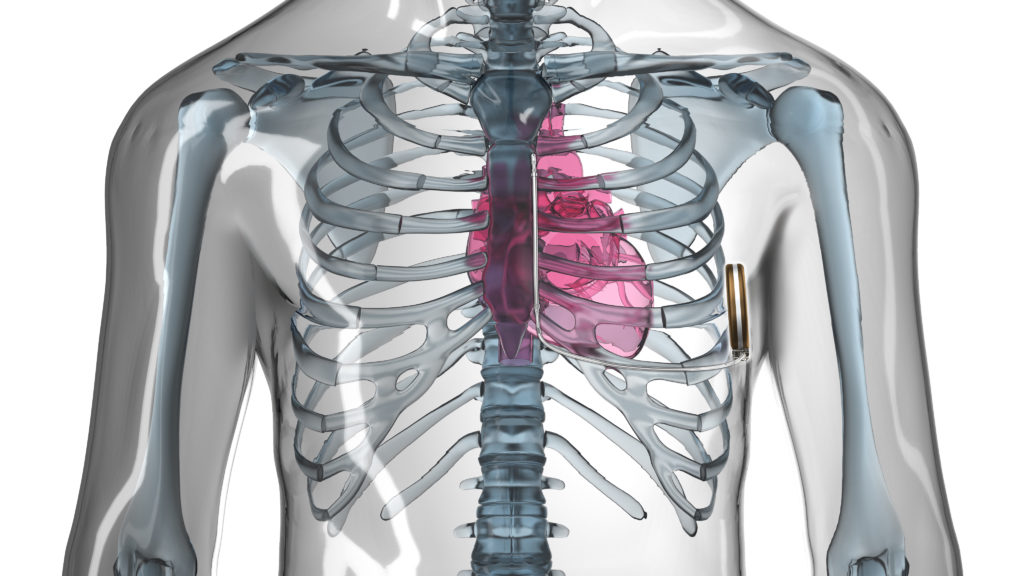
The implantable cardioverter defibrillator (ICD) is a well-established therapy for the prevention of sudden cardiac death. However, it is still associated with complications over time to the patient, leading to re-operation rates of 15.5% at six years.1 Many of the re-operation rates are due to the in-dwelling lead(s) consequent to mechanical breakdown and infection. Infection-related complications associated with cardiac devices have particularly high mortality rates at one year.2 The long-term weakness of cardiac lead systems has made a compelling case for the entirely subcutaneous ICD (S-ICD, Boston Scientific). Still, a considered weakness of the S-ICD is its inability to pace the myocardium for extremes of bradycardia or tachycardia; writes Martin C Burke (Chicago, USA).
The S-ICD does have a transthoracic pacing function between the shocking coils post-shock that paces at a rate of 50ppm for 30 seconds. The utility of this function is minor given the patient selection for the S-ICD and the pain related to its use. Advancing the S-ICD therapy as a platform for the future will require clever painless pacing modalities among other monitors that can provide bradycardia and tachycardia therapy with minimal invasion of the vasculature. As a start, combining the leadless cardiac pacemaker (LCP) with the S-ICD seems iteratively natural.3 We continue to explore the feasibility and functionality of combining these two independent modules of therapy through conductive radiofrequency communication in order to advance more tolerable bradycardia and anti-tachycardia pacing with an S-ICD.

Currently, the combination of the LCP and S-ICD requires device-device communication allowing delivery of appropriately timed sensing and pacing by using the individual strengths of both devices. Tapping into these strengths allows for less re-engineering of either device to accomplish the need. The strength of the first generation LCP is pacing without a lead. The strength of the S-ICD is sensing and treating successfully ventricular tachy-arrhythmias (VT) from the subcutaneous space. The ability to communicate in a near field body network is the key feature to leverage their working together with the least amount of energy resource. Importantly, creating communication at least unidirectional (S-ICD to LCP) poses a question, will combining the two devices’ functions through radiofrequency conductive signals during VT sensed events lead to disruptions in the main function of each individual device? In other words, what are the costs of combination if successful? Our research in acute and chronic animal studies suggests high success of communication with little risk to the patient, the function of either device or the successful treatment of VT.
Medical body networks have been predominantly wide area (ie. hospital telemetry) since their inception. The typical issue with Wi-Fi or wide area networks is the high energy resources needed. Progressive improvements, in near field communication and processor speed, now allow for low energy use to provide inductive and conductive delivery to imbedded materials in the body.4 The LCP and S-ICD modules are near field devices in the body. We have demonstrated successful unidirectional communication 98% of the time between an S-ICD and an LCP with as little as two conductive impulses at two volts in a near field across the chest.5 This type of communication allows for commanded anti-tachycardia pacing to terminate re-entrant VT from an S-ICD sensing device to an LCP treating device. In the future, bidirectional communication will expand the concept of modular devices (both therapeutic and diagnostic) to be added only when it will benefit the patient’s individual clinical needs rather than have all the features available but never utilised.
The next stage of cardiac implantable electronic device therapy is modular. The work performed so far is only the beginning. Adding no or minimal vascular modules in steps can limit the patients’ exposure to mechanical breakdown and chronic indwelling vascular lead infections. It allows for simplification and tailored device therapy to patients of all ages. Additionally, modular device therapy that uses medical body networks will also usher in the age of artificial intelligence where the S-ICD can act as the “mother ship” of energy resource and clinical decision making. The S-ICD as an implant platform will be the main information intake module processing the electrocardiographic, haemodynamic and genetic signals communicated from cleverly placed modules to indicate the timing and type of therapy that will have the best outcome for patients living with dynamic and life-threatening cardiovascular disease.
References:
- Ranasinghe I et al. Circulation 2014; 130:A20158
- Tarakji KG et al. Europace 2014; 16:1490‒5
- Tjong FV et al. Europace 2016; 18(11):1740‒1747
- Fang G, Orgun MA, Shankaran R, Dutkiewicz E, Zheng G. Truthful Channel Sharing for Self- Coexistence of Overlapping Medical Body Area Networks.
- Burke MC et al. Acute and chronic performance of communicating leadless anti-tachycardia pacemaker and subcutaneous implantable cardioverter defibrillator. Heart Rhythm Congress 2016; Birmingham, UK.
Martin C Burke is chief scientific officer, CorVita Science Foundation, Chicago, USA. His research on this subject is supported by a grant from Boston Scientific. He is a consultant for Boston Scientific and receives honoraria for speaking on behalf of the entirely subcutaneous ICD (S-ICD).
Click here to watch a presentation from Burke on this subject at the Heart Rhythm Congress (9‒12 October, Birmingham, UK).












