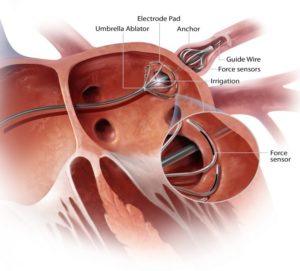
 AblaCor medical corporation has announced that it has received a notice of allowance from the United States Patent & Trademark Office on five additional patents for its CircumBlator AFib catheter ablation system to advance pulmonary vein isolation ablation procedures for patients with atrial fibrillation—the most common irregular heart rhythm and a potentially deadly disorder. When issued, AblaCor will have a total of nine patents issued for its CircumBlator system, with several more pending. Although AblaCor’s technology is designed to address atrial fibrillation, the patent portfolio also has been developed to include additional medical procedures.
AblaCor medical corporation has announced that it has received a notice of allowance from the United States Patent & Trademark Office on five additional patents for its CircumBlator AFib catheter ablation system to advance pulmonary vein isolation ablation procedures for patients with atrial fibrillation—the most common irregular heart rhythm and a potentially deadly disorder. When issued, AblaCor will have a total of nine patents issued for its CircumBlator system, with several more pending. Although AblaCor’s technology is designed to address atrial fibrillation, the patent portfolio also has been developed to include additional medical procedures.
“Atrial fibrillation is a major international epidemic,” said Martin Sklar, chief executive officer of AblaCor. “CircumBlator is designed to overcome the drawbacks of other ablation technologies due to their unpredictability in producing durable, continuous, transmural lesions. In fact, recurrence of atrial fibrillation after treatment with currently available technologies is reported in the scientific literature to be approximately 30 percent.”
AblaCor has also announced that it has established a Scientific Advisory Board with the appointment of prominent electrophysiologist Lawrence Rosenthal, who is director of Electrophysiology Service and professor of Medicine, University of Massachusetts Memorial Medical Center (Boston, USA). “Ablacor’s novel approach of a circular ablator, with its controlled electrode-tissue contact, designed to generate continuous and transmural ablation lesions reliably, holds great promise and would be a major advance,” said Dr. Rosenthal.
CircumBlator is designed to reduce or eliminate the 30% failure rate of existing pulmonary vein isolation procedures for treatment of stroke-causing and life-debilitating atrial fibrillation, an industry currently generating US$1.2 billion in medical device sales treating 300,000 patients annually.
CircumBlator’s innovative anchoring technology is designed to ensure that the ablation device is secured against the atrial wall during the entire ablation procedure, thus overcoming a key issue associated with the 30% failure rate of currently marketed products.









