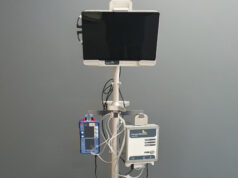
 SoloPace Incorporated has announced both US Food and Drug Administration (FDA) clearance and first-in-human use of its SoloPace control system for temporary pacing in transcatheter aortic valve implantation (TAVI) procedures.
SoloPace Incorporated has announced both US Food and Drug Administration (FDA) clearance and first-in-human use of its SoloPace control system for temporary pacing in transcatheter aortic valve implantation (TAVI) procedures.
With standardised workflows, the control system is engineered to improve TAVI temporary pacing efficiency and reduce patient risks, according to a recent company press release.
Initial cases were completed this month at Scripps Clinic in San Diego, USA by Paul Teirstein and Curtiss Stinis, the release adds.
“SoloPace has modernised pacing during TAVI valve deployment,” said Teirstein. “The device takes variability out of the procedure by giving the operator full control while automating ramp-up and back-up algorithms. The TAVI procedure is simplified, reducing physician and staff workload. We preprogramme pacer settings to reliably deliver the same procedure every time. This allows us to focus on the patient and free up resources—especially at centres where nurses and technologists currently control the pacemaker generator.”
The SoloPace control system’s operator-specific protocols and automated pace capture checks may improve procedural efficiency, while the intuitive interface and sterile remote-control operation are designed to reduce unnecessary communications errors, the company further claims. In addition, the system is compatible with patient cables for all common procedural pacing methods, making it easy to integrate into current practice.
“Precise pacing is essential for safe TAVI procedures, yet today’s devices are not purpose-built for TAVI, offer no standard presets, and require inefficient verbal commands,” stated SoloPace founder and chief executive officer (CEO) David Daniels. “FDA clearance and the use of our technology in patients are both significant milestones for the company, and first steps on our journey to advance transcatheter heart valve pacing solutions.”