 Cardiologs has announced the launch of a clinical study using the company’s artificial intelligence (AI) solution to remotely monitor the cardiac safety of COVID-19 patients during hydroxychloroquine treatment via analysis of electrocardiogram (ECG) data gathered from smartwatches.
Cardiologs has announced the launch of a clinical study using the company’s artificial intelligence (AI) solution to remotely monitor the cardiac safety of COVID-19 patients during hydroxychloroquine treatment via analysis of electrocardiogram (ECG) data gathered from smartwatches.
Hydroxychloroquine therapy is associated with the risk of QT prolongation, a specific sign found on ECGs, which can lead to severe cardiac arrhythmias.
The trial will study patients with COVID-19 at the University Hospital of Marseille (France) who are being treated with hydroxychloroquine and azithromycin, a drug combination currently being evaluated as a therapy for the coronavirus (SARS-CoV-2). These drugs can cause QT prolongation as a side effect, especially when used together, raising concerns about the risk of arrhythmic death.
“A significant QT prolongation can lead to ventricular arrhythmia and potentially deadly consequences” explained Laurent Fiorina, a cardiologist at the Institut Cardiovasculaire Paris Sud (ICPS) and a medical expert at Cardiologs who initiated the project. “It is thus important to closely monitor the QT interval during this treatment.”
ECG assessment to monitor the QT interval is the current standard of care to ensure cardiac safety in clinical settings. With COVID-19, monitoring is especially challenging to implement because of the risk of contamination. It is also highly unlikely that it could be applied as a standard to a large population within a short period of time because of the strain on hospital resources.
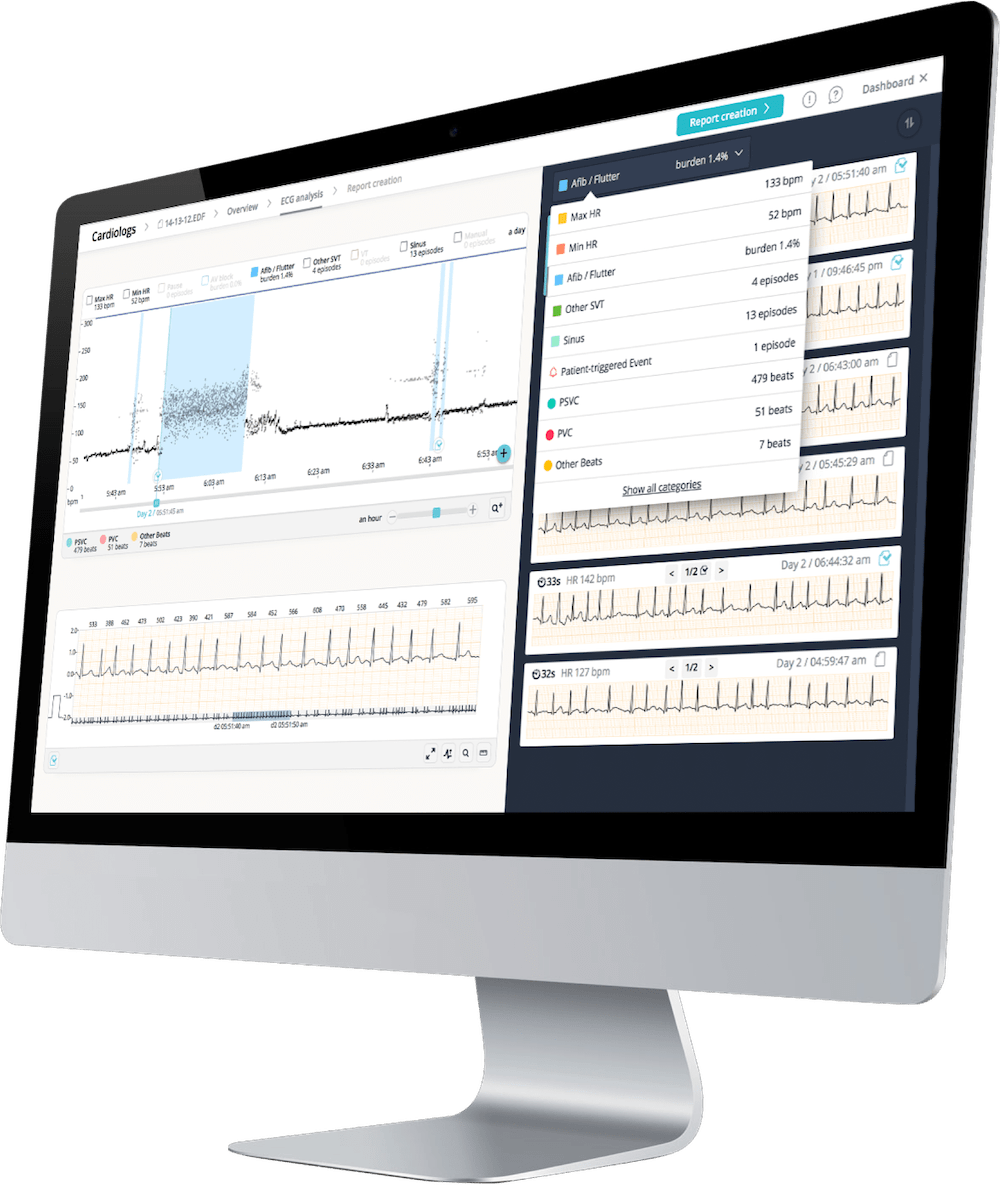
“The objective of our study is to evaluate a new method for QT measurement using Cardiologs’ AI-based solution and ECG data collected via smartwatches,” added Jean-Claude Deharo, head of the cardiac arrhythmia department at the University Hospital of Marseille (France) and the principal investigator of the study. “Smartwatches are already used in the clinical setting but do not have validated QT analysis available. Combining these technologies will enable clinicians to overcome the practical limitations in the context of COVID-19 of the standard cardiac safety strategy that requires heavy patient interaction.”
Each patient in the study will receive a Withings Move ECG Watch linked to Cardiologs’ AI platform, which is already CE marked and US Food and Drug Administration (FDA) cleared for QT interval analysis and arrhythmia diagnostics. Throughout the 10-day course of treatment, daily ECG readings will be sent directly from the smartwatch to Cardiologs’ AI platform, where the data will be compared to the gold standard ECG measure and analysed to assess drug-induced cardiotoxic risk as well as arrhythmic events.
“This study has implications for risk management of drug-induced cardiotoxicity, even beyond the current COVID-19 and hydroxychloroquine context,” said Jag Singh, a cardiologist at Massachusetts General Hospital (Boston, USA), professor of medicine at Harvard Medical School, and a scientific advisor to Cardiologs. “Personal ECG sensors could potentially find a role in the management of these patients, but also add value in other routine clinical care, since over 300 commonly used drugs may have similar QT-prolongation risks as hydroxychloroquine.”
Cardiologs is exploring the possibility of additional studies involving different wearable ECG monitors with partners in the USA.