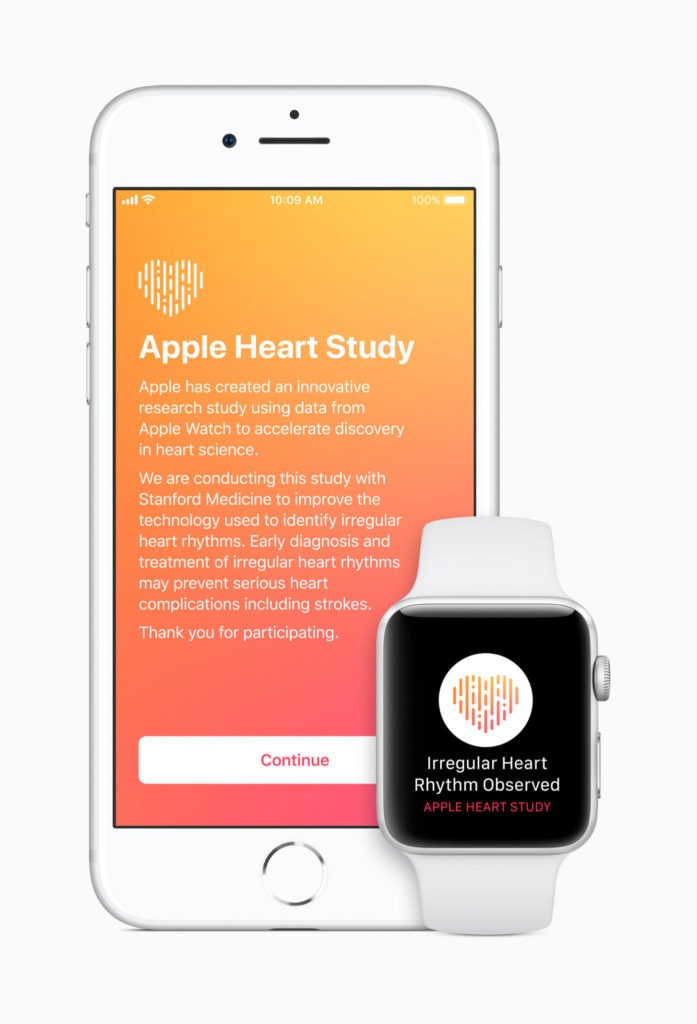
 Apple has launched the Apple Heart Study app, a first-of-its-kind research study using Apple Watch’s heart rate sensor to collect data on irregular heart rhythms and notify users who may be experiencing atrial fibrillation (AF).
Apple has launched the Apple Heart Study app, a first-of-its-kind research study using Apple Watch’s heart rate sensor to collect data on irregular heart rhythms and notify users who may be experiencing atrial fibrillation (AF).
To calculate heart rate and rhythm, the Apple Watch uses rapid flashing green LED lights and light-sensitive photodiodes to detect blood flow through the wrist. The sensor’s optical design gathers signals from four distinct points on the wrist, and the watch then isolates heart rhythms from other noise with algorithms. The Apple Heart Study app uses this technology to identify an irregular heart rhythm.
“Working alongside the medical community, not only can we inform people of certain health conditions, we also hope to advance discoveries in heart science”, says Jeff Williams, Apple’s chief operating officer.
Apple is partnering with Stanford Medicine to perform the research. As part of the study, if arrhythmia is identified, participants will receive a notification on their Apple Watch and iPhone, a free consultation with a study doctor and an electrocardiogram (ECG) patch for additional monitoring. The Apple Heart Study app is available in the US App Store to customers who are 22 years or older and have an Apple Watch Series 1 or later.
“Through the Apple Heart Study, Stanford Medicine faculty will explore how technology like Apple Watch’s heart rate sensor can help usher in a new era of proactive health care central to our Precision Health approach,” said Lloyd Minor, Dean of Stanford University School of Medicine. “We’re excited to work with Apple on this breakthrough heart study.”
Doctors and medical researchers around the world have been using iPhone and Apple Watch to revolutionise medical studies. Apps created with Apple’s ResearchKit platform, a software tool researchers use to conduct studies, have produced new insights and discoveries about conditions like autism and Parkinson’s disease. To date, Apple’s ResearchKit and CareKit platforms have been used by over 500 researchers and more than three million participants.
The news comes shortly after the US Food and Drug Administration (FDA)
released its first medical device clearance for an Apple Watch accessory: the KardiaBand (AliveCor), an ECG monitor strap, which similarly works with an app to detect arrhythmia.
 Apple has launched the Apple Heart Study app, a first-of-its-kind research study using Apple Watch’s heart rate sensor to collect data on irregular heart rhythms and notify users who may be experiencing atrial fibrillation (AF).
Apple has launched the Apple Heart Study app, a first-of-its-kind research study using Apple Watch’s heart rate sensor to collect data on irregular heart rhythms and notify users who may be experiencing atrial fibrillation (AF).








