 Biosense Webster has announced the release of the Octaray mapping catheter, developed for the mapping of cardiac arrhythmias including atrial fibrillation (AF). The catheter comes with TRUEref technology— a novel mapping reference electrode—powered by the Carto 3 version 7 system.
Biosense Webster has announced the release of the Octaray mapping catheter, developed for the mapping of cardiac arrhythmias including atrial fibrillation (AF). The catheter comes with TRUEref technology— a novel mapping reference electrode—powered by the Carto 3 version 7 system.
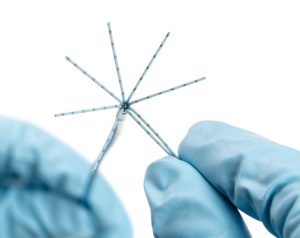
In a press release, Biosense Webster details that Octaray has eight splines with “improved electrode spacing options” to provide shorter and more efficient mapping times than the Pentaray Nav Eco mapping catheter, which may shorten overall ablation procedure times.
Catheter ablation is a safe and effective procedure to restore the heart’s incorrect electrical signals, which causes an abnormal heart rhythm. The Octaray mapping catheter can map arrhythmias in any chamber and provides physicians with enhanced clarity, speed and integration to quickly capture precise information for their catheter ablation procedures.
“With more splines and electrodes, the increased surface area coverage and improved signal quality with the Octaray mapping catheter allows me to better understand the anatomy and conduction properties of the chamber of interest,” said Amit Thosani (Allegheny Health Network, Pittsburgh, USA). “This catheter not only helps me to map more accurately and efficiently, but also allows for better patient specific therapy.”
The catheter has 48 small mapping electrodes on eight splines, reduced electrode size and tight electrode spacing.
“I am excited about the addition of the Octaray mapping catheter to the suite of tools available to map cardiac arrhythmias at my institution,” said Benjamin Berte (Cantonal Hospital, Lucerne, Switzerland). “As the prevalence of patients with AF continues to rise, physicians need innovative tools to deliver more efficient and effective procedures to benefit their patients.”
The Octaray mapping catheter with TRUEref technology is now available in the USA and EMEA and has been both CE marked and 510(k) cleared by the US Food and Drug Administration (FDA).









