Boston Scientific recently announced late-breaking data from the MODULAR ATP clinical trial investigating the pacing performance of the company’s Empower leadless pacemaker. The data were delivered at the 2024 European Society of Cardiology (ESC) congress (30 August–2 September, London, UK) in a presentation titled, “Pacing performance of the first leadless pacemaker communicating with an S-ICD [Subcutaneous implantable cardioverter defibrillator] from the full cohort of the MODULAR ATP study”.
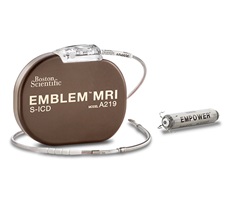
MODULAR ATP is a clinical trial of the modular cardiac rhythm management (mCRM) system—a system that consists of the Emblem S-ICD device and the Empower leadless pacemaker, which work together wirelessly to coordinate painless intracardiac anti-tachycardia pacing (ATP) therapy, provide rate-responsive bradycardia pacing support, and prevent sudden cardiac death without the risk of leads in the heart or under the sternum.
The recent presentation at ESC 2024 focused on outcomes of the 293 patients enrolled in the MODULAR ATP study as of 24 January 2024, and evaluated the Empower leadless pacemaker’s performance in rate-adaptive pacing, and pacing in the ambulatory setting, respectively.
Results from the study demonstrated a high rate of successful leadless pacemaker implantation with few complications and stable pacing parameters, according to Boston. A recent press release from the company details that a rate-response sub-study (n=35 patients) measured the leadless pacemaker’s accelerometer response to support rate-adaptive pacing. Another 31 patients underwent Holter monitoring to confirm ambulatory performance.
In addition, pacing performance measures were stable and within acceptable ranges. Accelerometer performance showed a mean metabolic-chronotropic relation (MCR) slope of 0.96 (95% confidence interval [CI], 0.91–1.02). The release goes on to state that the Holter sub-study showed appropriate pacing behaviour and that, at six months, 97% of patients had a pacing burden ≤10%.
“We saw excellent overall clinical performance of the Empower leadless pacemaker in the MODULAR ATP trial, including a high rate of successful implants, few complications and stable pacing parameters,” said presenting author Lluis Mont (University of Barcelona, Barcelona, Spain). “These findings indicate that the device can function as a standalone pacemaker to provide rate-responsive bradycardia pacing to patients, and complement data previously published from this study, which showed a high percentage of pain-free termination of spontaneous tachyarrhythmia episodes when used in connection with the Emblem S-ICD.”
“Successful performance of the Empower leadless pacemaker in the MODULAR ATP study provides crucial evidence that it will function as a standalone pacemaker to treat bradycardia, in addition to its anti-tachycardia pacing capabilities when used in connection with the Emblem S-ICD,” said Kenneth Stein, senior vice president and global chief medical officer at Boston. “We believe this device will provide additional options for physicians to treat cardiac arrhythmias, without subjecting patients to the risks of more invasive, lead-based approaches—a capability that will make it a strong addition to the Boston Scientific portfolio.”








