This case report has been written by Timothy Betts (consultant cardiologist and electrophysiologist, Oxford University Hospitals NHS Foundation Trust, Oxford, UK) and has been sponsored by EBR Systems.

Conventional cardiac resynchronisation therapy (CRT) is a widely accepted treatment option for patients with heart failure, impaired left ventricular systolic function and electrical interventricular dyssynchrony. Despite advances in technology, the ability to place a pacing lead in a left ventricular coronary vein is unsuccessful in up to 5% of patients due to constraints of the coronary sinus anatomy, high pacing thresholds or phrenic nerve stimulation. Lead instability and dislodgment post-procedure prevent adequate biventricular pacing in a further 10% of patients. Even in the absence of technical issues, the clinical response to conventional CRT remains highly variable, with up to one-third of patients not responding. A commonly cited reason is the inability to select an appropriate pacing site with lead positions constrained by suboptimal venous anatomy. In the effort to overcome these limitations, alternative methods of CRT delivery have been developed.
WiSE CRT in a nutshell
The WiSE (Wireless Stimulation Endocardially) CRT technology is a unique and innovative approach, delivering wireless left ventricular (LV) endocardial pacing as an alternative to conventional epicardial LV pacing through the coronary veins. It comprises a battery, an ultrasound transmitter and a receiver electrode and is combined with a pre-existing right ventricular (RV) pacing device. The battery, implanted subcutaneously in the left mid-axillary line, powers the ultrasound transmitter which is inserted in an intercostal space below the pectoralis muscle. Pre-implant screening is used to identify the optimal site for the transmitter; one that provides an acoustic window to the posterior LV that is not impeded by lung tissue. The transmitter synchronises with the RV pacing pulse and immediately transmits ultrasound energy to a tiny receiver electrode implanted on the LV endocardial surface. The receiver electrode converts ultrasound energy into electrical energy, providing LV stimulation within two milliseconds of RV stimulation, resulting in simultaneous biventricular pacing.
Clinical evidence and benefits
The recent WiSE-CRT and SELECT-LV studies have confirmed the feasibility of the WiSE CRT system, with achievement of CE mark in October 2015. The preliminary efficacy data from the SELECT-LV study are very encouraging, showing achievement of CRT in 97% of patients with a previous failed CRT at one month post-implant and sustained cardiovascular improvement at six months in 85% of patients.
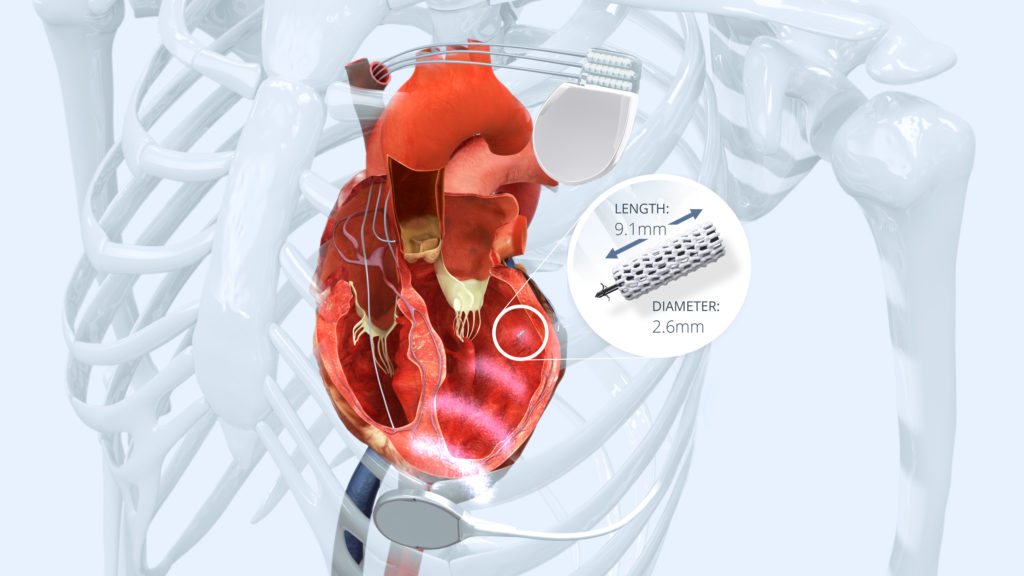
Wireless pacing provides a simple alternative to surgical epicardial lead placement after failed CRT procedures, or when there is no venous access and lead extraction is considered too risky. For non-responders, particularly those with a lead in a suboptimal coronary vein, the ability to select a more advantageous anatomical pacing site with a longer stimulus ‒ LV time and better resynchronisation may offer acute haemodynamic benefit and subsequent functional response. It is also indicated for upgrading pacemakers or implantable cardioverter defibrillators (ICDs) to CRT-equivalent devices. There is also growing evidence that the endocardial LV pacing could be superior to epicardial pacing by reproducing the physiological endo-epicardial activation and systolic contraction of the left ventricle, more rapid myocardial recruitment and maximised contractile response. Unlike transvenous endocardial pacing through the interatrial or interventricular septum, wireless pacing with the WiSE CRT system does not require systemic anticoagulation and does not increase thromboembolic risk.
Case
A 76-year-old man was referred to our institution for an upgrade of his dual-chamber pacemaker to a CRT pacemaker. He had a past history of complete heart block requiring a dual chamber permanent pacemaker in 2009 and a more recent diagnosis of dilated cardiomyopathy with an LV ejection fraction of 30% and New York Heart Association (NYHA) class III.
A pre-procedural left-sided venogram demonstrated total occlusion of the left subclavian vein with multiple collaterals. The options of a right-sided approach with the left ventricular lead tunnelled across to the left-sided pocket, a full extraction and replacement of the left-sided system, a surgical epicardial LV lead placement or a WiSE CRT system were discussed with the patient, before deciding on a WiSE CRT system.
Pre-implant echocardiographic screening identified a suitable acoustic window. On day one, under general anaesthesia, the ultrasound transmitter was inserted into the fifth intercostal space above the intercostal muscle. The connecting cable was tunnelled to the battery pack which was inserted subcutaneously in the left axilla.
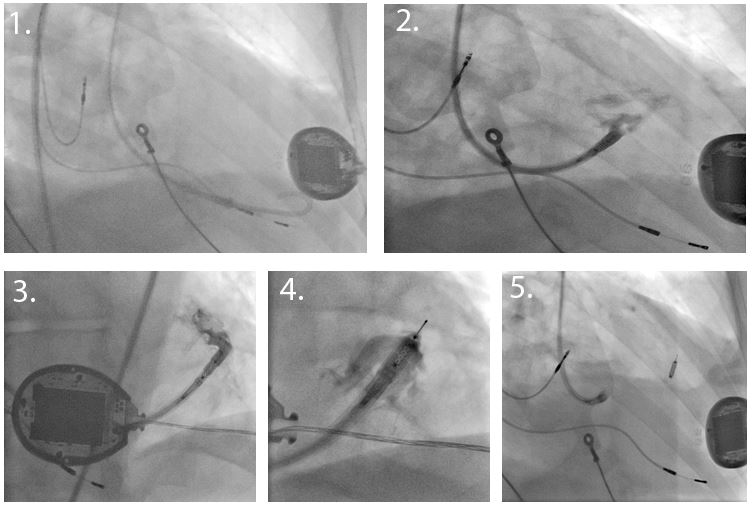
On the second day, under light sedation and local anaesthesia, a retrograde aortic approach via the femoral artery was used (Figure 1). The 12F deflectable sheath with an inflatable atraumatic balloon at the distal tip was introduced into the LV over a pigtail catheter and guidewire. An 8F electrode catheter was inserted into the delivery sheath and advanced to the distal end. The electrode was tested at multiple sites, selecting a position with optimal electrical parameters including long stimulus-LV timing, low threshold, no latency and a narrow QRS. The receiver electrode was then deployed into the LV endocardium whilst using gentle puffs of radiopaque contrast injected through the catheter in order to confirm the correct anchoring. The electrode was then detached and released from the delivery system.
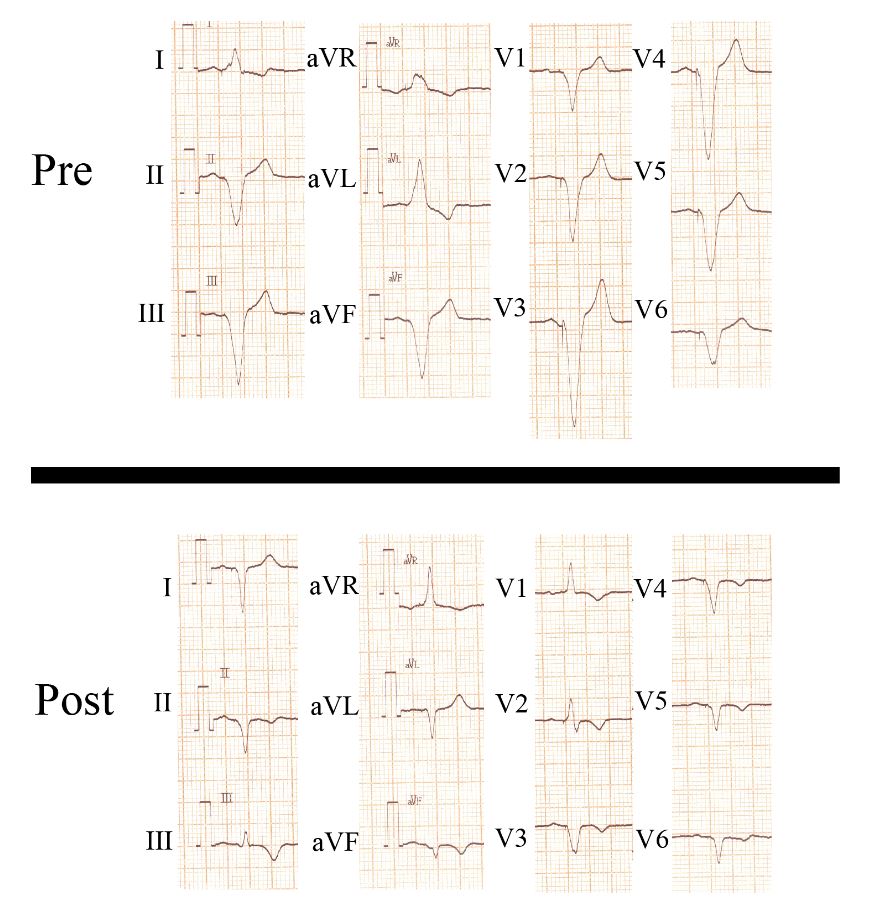
A site with late electrical activation from the right ventricular pacing stimulus (Q-LV 135 milliseconds) within the optimal range of the transmitter and a pacing threshold of less than one volt was chosen. Biventricular pacing was associated with a 44 millisecond reduction in QRS duration (from 168 to 124 milliseconds) with a dominant R-wave in lead V1 (Figure 2).
At three-month follow-up, the WiSE CRT system demonstrated satisfactory pacing parameters, a >99% biventricular pacing rate and the patient referred a symptomatic improvement corresponding to a current NYHA class of II.









