Bruce Wilkoff (Cleveland Clinic, Cleveland, USA) presented results of the worldwide randomised antibiotic envelope infection prevention trial (WRAP-IT) to reduce CIED infection at the 2019 annual meeting of the European Heart Rhythm Association (EHRA; 17–19 March, Lisbon, Portugal).
The investigators found that in patients undergoing any CIED generator replacement, system upgrade, or revision of initial CRT-D implantation, the rate of major CIED infections was 1.2% at one-year, the TYRX envelope significantly reduced major CIED infection by 40%, without increasing complications, and major pocket infections were reduced by 61%.
According to Wilkoff, “This study provides comprehensive data on CIED infection and strong evidence for the use of the TYRX envelope for infection in this patient population.”
The goal of the WRAP-IT study was to evaluate the safety and effectiveness of the TYRX absorbable antibacterial envelope in reducing CIED infections and the primary endpoint was rate of major CIED infections through 12-months.
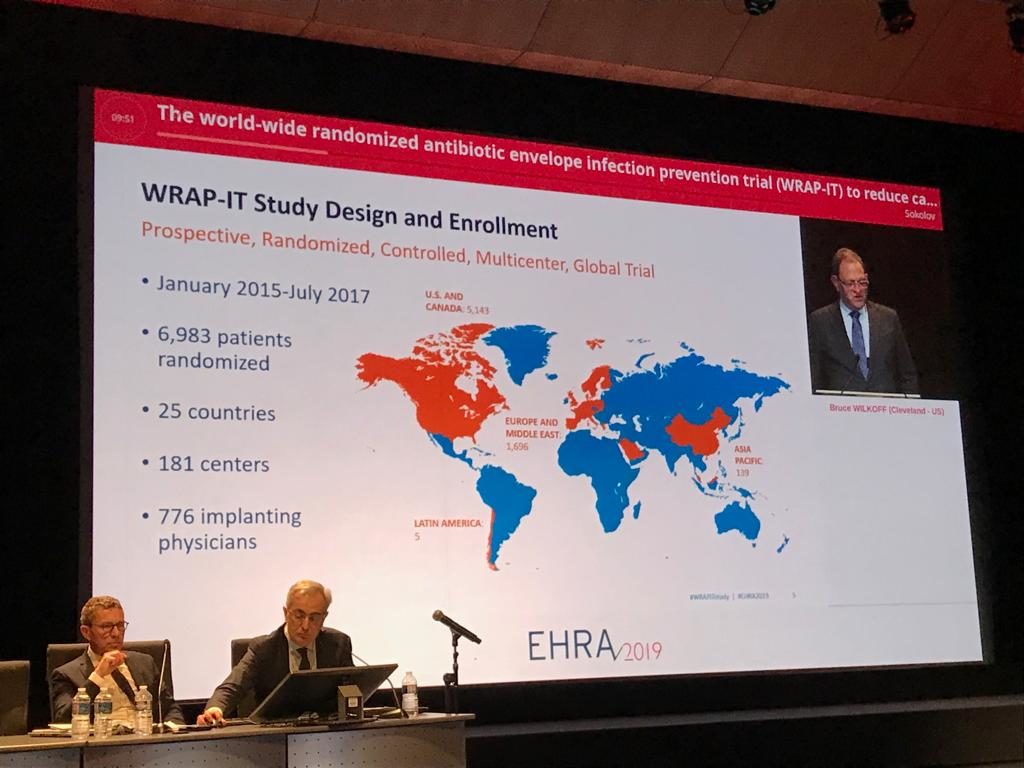
Wilkoff described the design of the study as a prospective, randomised, controlled, multicentre, global trial, which included 6,983 patients in 181 centres across 25 countries. The trial involved 776 implanting physicians and took place between January 2015 and July 2017.