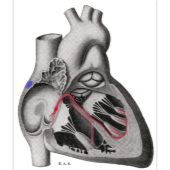
A press release has announced US Food and Drug Administration (FDA) labeling expansion for the Medtronic SelectSecure MRI SureScan Model 3830 cardiac pacing lead to include stimulation of the bundle of His. It is the only pacing lead on the market approved for His-bundle pacing (HBP).
The steroid-eluting, bipolar SelectSecure SureScan MRI model 3830 lead (lengths of 59, 69 and 74 cm) is attached to a single or dual chamber pacemaker, and approved for pacing and sensing in the atrium, right ventricle, or at the bundle of His as an alternative to right ventricular pacing. Lead placement at or near the bundle of His can be facilitated by the Medtronic C315HIS delivery catheter. Permanent His bundle pacing is a physiologic alternative to right ventricular pacing, harnessing the heart’s native His-Purkinje system.
“There is growing interest among some physicians in His-bundle pacing as an alternative to right ventricular pacing, and we are pleased to offer the SelectSecure MRI lead for those who would like the option of this pacing approach for appropriate patients,” said Rob Kowal, vice president and chief medical officer of the Cardiac Rhythm and Heart Failure division, which is part of the Cardiac and Vascular Group at Medtronic.









