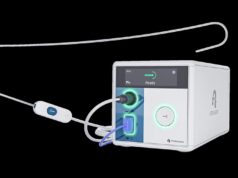
Atraverse Medical has announced US Food and FDA clearance of its Hotwire radiofrequency (RF) guidewire, a novel device that enables zero exchange left-heart access while also acting as a rail for catheter-based therapy systems.
Atraverse Medical has announced US Food and FDA clearance of its Hotwire radiofrequency (RF) guidewire, a novel device that enables zero exchange left-heart access while also acting as a rail for catheter-based therapy systems.
This marks a significant step towards commercialising the company’s best-in-class left-heart access technology, Atraverse Medical said in a press release.
The Hotwire system features universal sheath compatibility and optimised RF technology, and aims to improve patient outcomes and streamline procedural workflows.
Steven Mickelsen, a cardiac electrophysiology physician at Scripps Hospital in La Jolla, USA, and co-inventor of the system, commented: “The FDA clearance of the Hotwire underscores our dedication to medical innovation and our commitment to improving the standard of care for procedures requiring transseptal access including endocardial ablation, left atrial appendage closure, and mitral valve repair.”
Atraverse Medical has also announced the oversubscription of its seed round. The company has secured US$12.5 million of total seed investment, demonstrating a strong investor belief in the need for continued innovation in the left-heart access arena.