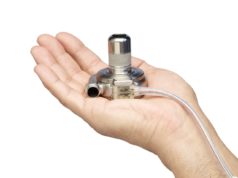
Medtronic has announced plans for a global registry of its HeartWare HVAD System, following the release of two-year outcomes from the LATERAL clinical trial. The HVAD System is a left ventricular assist device (LVAD) that helps increase the amount of blood that circulates through the body in patients with advanced heart failure.
According to a press release from the company, LATERAL evaluated the use of HeartWare in patients who received the system via a less-invasive, thoracotomy implant approach. The data showed that after two years of follow up, 95% of HVAD patients implanted via thoracotomy were free from disabling stroke (Modified Rankin Score [mRS] >3).
Additionally, the press release states, a review of adverse events occurring in the LATERAL trial revealed that they were more likely to occur during the first 30 days after implant, with a significant decline in bleeding (1.53 vs. 0.51 events per patient year [EPPY], p<0.001), arrhythmias (3.22 vs. 0.26 EPPY, p<0.001) and strokes (0.51 vs. 0.12 EPPY, p=0.01) in the later time periods (>30 days–180 days) that patients are on LVAD support. Overall, adverse event rates were meaningfully reduced one to six months post-implant.
The press release also says that the late risk of stroke was very low, with total strokes occurring at only 0.05 EPPY in years one to two post-implant.
The global registry aims to collect additional clinical evidence to further characterise survival, adverse events, and economic benefits of the HVAD System when implanted via a lateral thoracotomy in bridge-to-transplant (BTT) or destination therapy (DT) patient populations. Together with the HVAD Destination Therapy Post Approval Study (DT PAS) and Apogee Study, both of which are prospective, observational, multisite HVAD studies, Medtronic says it aims to advance physician knowledge and patient access to pump therapy.
The HVAD System is currently available in 56 countries.