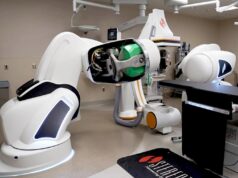
Medtronic has announced today that it has received CE Mark for the Affera mapping and ablation system, which includes the Sphere-9 catheter and the Affera Prism-1 mapping software.
The full system creates a new paradigm in electrophysiology through the unique integration of the Sphere-9 pulsed field ablation (PFA), radiofrequency (RF), and high density (HD) mapping catheter, which maps and ablates atrial arrhythmias (fast, abnormal heart rhythms) and provides real-time feedback through its intuitive mapping and navigation software.
Atrial fibrillation (AF) is the most common atrial arrhythmia, affecting close to 60 million people worldwide, with a further five million patients expected to join this total figure every year by 2030. Atrial arrhythmias, such as AF, are associated with serious complications including heart failure, stroke and increased risk of death.
The Sphere-9 catheter, coupled with the integrated mapping and navigation system, generates sophisticated electro-anatomical maps allowing the physician to deliver wide-area focal ablation lesions of choice between RF or PFA, based on the patient and procedure needs.
The all-in-one catheter’s nitinol 9mm ablation tip has the potential to require fewer focal ablation lesion applications that may result in lower procedure times than standard irrigated ablation catheters. The intuitive mapping software enables an optimised user experience by delivering streamlined insights and feedback to support procedure performance.
“The revolutionary Affera mapping and ablation system combined with the novel Sphere-9 catheter represent a great advancement in the field of HD mapping and focal ablation,” said Khaldoun Tarakji, Medtronic vice president of Cardiac Ablation Solutions business, which is part of the cardiovascular portfolio at Medtronic.
Tarakji continues, stating: “Current technologies require the use of separate HD mapping and ablation catheters. The ability to map, ablate, and validate with the Sphere-9 catheter enables the physician to eliminate the need to exchange catheters and empowers them to choose the energy source, whether RF or PF, based on the patient’s needs. All this leads to improving efficiency and most importantly, enhancing the safety of ablation procedures for our patients.”
Supported by results from clinical studies assessing the safety and performance of the Sphere-9 catheter and mapping system, CE Mark approval comes on the heels of a December 2022 announcement that enrolment was completed in the Affera SPHERE Per-AF clinical trial, a randomised, controlled US Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal trial. Designed to evaluate the safety and effectiveness of the Affera system for the treatment of persistent atrial fibrillation, the SPHERE Per-AF IDE trial is currently in its 12-month follow-up phase.
“Electrophysiology is evolving at a rapid pace, and we believe we are uniquely positioned to be category creators once again with the all-in-one Sphere-9 catheter, just as we did when Medtronic pioneered cryoablation technology,” said Rebecca Seidel, president, Cardiac Ablation Solutions. “Along with the PulseSelect PFA system, we are proud to be among the first to bring novel single shot and focal PF technologies to patients around the world.”