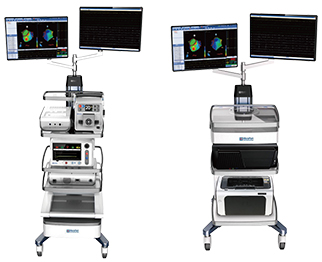
MicroPort EP has recently received approval from the China Food and Drug Administration (CFDA) for Columbus 3D EP navigation system and FireMagic 3D irrigated ablation catheter. The approval of these products marks their official entrance to the Chinese market.
Columbus, designed to feature real-time electromagnetic device tracking with cardiac motion compensation, is intended to offer 3D simulation of the catheter deflectable segment and geometric reconstruction of cardiac chambers. According to a press release, it also features an integrated echocardiogram recording module, 3D image segmentation of cardiac chambers, and preoperative computed tomography image registration, as well as integration.
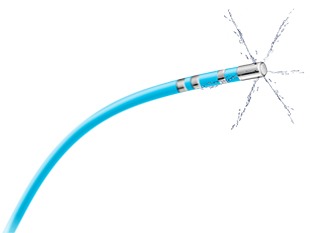
The FireMagic catheter is offered in two types: six-hole irrigation (FireMagic Cool 3D) and micro-hole irrigation (FireMagic SuperCool 3D), which is intended to cool the ablation electrode more uniformly.
These products were approved, according to the release, based on the clinical trials in China that enrolled 255 patients with persistent atrial fibrillation (treatment group of 167 patients and control group of 88 patients) in 13 Chinese EP centres, and showed no difference from other approved products on the market in treating complex arrhythmias.
“Columbus’ customised design simplifies the interface. Apart from accurate localisation, Columbus also does a good job in heart chamber reconstruction,” says Changsheng Ma, director of Cardiology Department, Beijing Anzhen Hospital, Capital Medical University (CMU), Beijing, China.
Columbus and FireMagic obtained the CE mark in November, 2013, and have been distributed in Europe and Latin America.









