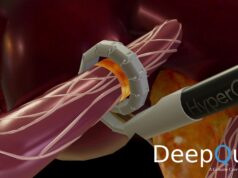
DeepQure has announced that South Korea’s Ministry of Food and Drug Safety (MFDS) has approved a clinical trial of the company’s HyperQure renal denervation (RDN) system—a novel laparoscopic RDN system for the treatment of atrial fibrillation (AF).
DeepQure has announced that South Korea’s Ministry of Food and Drug Safety (MFDS) has approved a clinical trial of the company’s HyperQure renal denervation (RDN) system—a novel laparoscopic RDN system for the treatment of atrial fibrillation (AF).
The trial is designed to evaluate the safety and efficacy of laparoscopic RDN in patients with recurrent AF following pulmonary vein isolation (PVI) and resistant hypertension, and will be conducted as a multicentre, prospective, single-arm, open-label exploratory study.
“This approval validates HyperQure as a next-generation solution that overcomes the limitations of traditional RDN techniques,” said a DeepQure spokesperson. “With parallel trials in resistant hypertension and now AF, we are accelerating our push into the global cardiovascular market with a truly differentiated solution.”
HyperQure is described by a company press release as a next-generation laparoscopic RDN device developed under the leadership of Chang-Wook Jeong at Seoul National University Hospital (Seoul, South Korea) over the past decade. By adopting an extravascular approach, HyperQure is said to address key limitations of conventional intravascular RDN, offering direct anatomical access to renal nerves with the potential for improved precision and outcomes.
DeepQure is also conducting RDN clinical trials for resistant hypertension in both South Korea and the USA. Patient enrolment is nearing completion in South Korea, while trials are actively underway at five major academic medical centres across the USA.