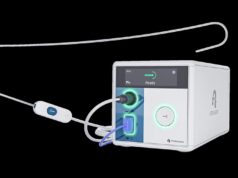
Stereotaxis has announced that regulatory submissions were made recently in both Europe and the USA for the MAGiC catheter. These submissions follow initial clinical results in an ongoing trial.
Stereotaxis’ MAGiC catheter is a robotically navigated magnetic ablation catheter designed to perform minimally invasive cardiac ablation procedures.
The first human procedures with the MAGiC catheter commenced earlier this year as part of a prospective multicentre clinical study. Initial results during the first 20 procedures were submitted for publication, with the authors documenting 100% acute efficacy and no adverse events. These results were included in the regulatory submissions.
The CE mark and premarket approval supplement submissions of the MAGiC catheter reflect the culmination of an extensive design, development, manufacturing and testing effort. The catheter is the first in a series of interventional devices being developed by Stereotaxis and serves as a platform for future innovations. Stereotaxis anticipates making the MAGiC catheter commercially available for the community of robotic electrophysiologists following receipt of regulatory clearances.
“We are excited to have reached this significant milestone for Stereotaxis and for the physician community that is pioneering the frontiers of robotics in electrophysiology,” said David Fischel, Stereotaxis chairman and CEO. “We look forward to working collaboratively with the regulatory agencies during their review of our submissions and hope to make the benefits of MAGiC available to patients and physicians in the near future.”