Tag: CE mark
Acutus Medical’s new contact mapping software receives CE mark
Acutus Medical recently announced CE mark approval for AcQMap contact mapping software, offering expanded functionality that provides physicians with more options to inform individualised...
CE mark for Watchman FLX left atrial appendage closure device
Boston Scientific announced it has received CE mark and initiated a limited market release of the next generation Watchman FLX left atrial appendage closure...
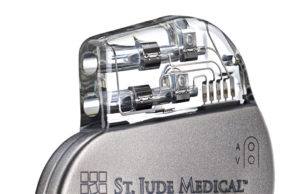
3T MRI CE mark announced for MRI-compatible pacemaker
Abott has announced Conformité Européenne (CE) Mark for 3 Tesla (T) magnetic resonance-conditional labelling for both the Assurity MRI pacemaker and the Tendril STS...
Boston Scientific launches Resonate CRT-D platform in Europe
Boston Scientific has launched the Resonate cardiac resynchronisation therapy defibrillator (CRT-D) systems in Europe. This new family of devices features SmartCRT technology, which recently...