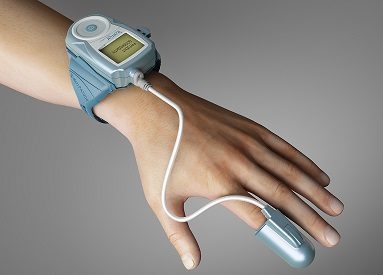
Itamar Medical has received US Food and Drug Administration (FDA) clearance to expand the medical indication of WatchPAT for sleep apnoea diagnosis. WatchPAT is a home sleep test diagnostic device for sleep apnoea.
Under this approval, the use of WatchPAT in the USA is permitted from the age of twelve, expanding the previous indication for ages seventeen and older. Similar approvals were recently granted in Japan and Europe.
David Gozal, a paediatric sleep physician and scientist at the University of Chicago and Comer Children’s Hospital in Chicago, USA, and the president of the American Thoracic Society, adds that “the clearance of the WatchPAT for clinical use in adolescents is a welcome addition to the currently limited array of home-based diagnostic technologies for children suspected to suffer from sleep-disordered breathing, and should enable reliable and more timely detection of those patients in need for treatment”.









