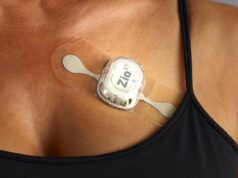
A phase 3 trial of Zoll Medical’s LifeVest has missed its primary endpoint. The study found that people who started using the wearable defibrillator the week after they had a heart attack did not experience a statistically significant reduction in sudden cardiac death in the next three months as patients who received conventional therapy; however, patients did experience a statistically significant reduction in the secondary endpoint of total mortality.
The investigators designed the 2,300-patient trial to show that the LifeVest reduces the sudden death rate by monitoring the wearer’s heart rhythms and delivering a treatment shock if they go awry. However, the study found the device did not statistically significantly reduce sudden death mortality. Investigators claim this result may be due to decreased power for the primary outcome of sudden death due to difficulty in misclassification of cause of death.
“While the VEST Trial did not meet the endpoint of sudden death mortality, the ability to determine the cause of death as sudden when unwitnessed is difficult and could result in misclassification,” Jeffrey Olgin, co-principal investigator of the trial, says as a potential explanation for not seeing a statistically significant reduction in sudden death.
Olgin’s assessment is supported by data from a secondary endpoint that looked at all-cause mortality. The all-cause mortality rate in the control arm was 4.9%, compared to 3.1% in the LifeVest cohort.That difference amounts to a 36% reduction in mortality and a p-value of 0.04, giving the wearable cardiac defibrillator (WCD) a data point that, while having delimited clinical significance, does not suggest the study was an outright failure and could make the point for further investigation.
The study concludes that the wearable cardioverter-defibrillator did not statistically significantly reduce sudden death mortality, but it did reduce total mortality in the first 90 days postmyocardial infarction in patients with left ventricular ejection fraction 35%, and there was a relative risk reduction of 35.5%.
Prescribing the wearable cardioverter-defibrillator is reasonable to protect high-risk patients with a low left ventricular ejection fracture postmyocardial infarction until evaluation for an implantable cardioverter defibrillator at 40–90 days.
The study only investigated the impact of the WCD in patients who suffered a recent heart attack (MI) and had a low ejection fraction: other indications (such as non-ischemic cardiomyopathy or patients with explanted ICDs) were not studied in this trial.
The study enrolled 2,302 post myocardial infarction patients (13,774 patients were screened) with low ejection fractions who were randomised in a 2:1 ratio to either receive a wearable cardiac device and optimal medical therapy (n=1,524) or optimal medical therapy alone (n=778). The patients were followed up after 90 days and they had a mean age of 61 years and were 27% female.
The inclusion criteria were being within seven days of hospital discharge for acute myocardial infraction and having an ejection fracture of less than 35%. The exclusion criteria were having an existing implantable cardioverter-defibrillator, significant valve disease, unipolar pacing system, chronic haemodialysis, a chest too small or large to wear the wearable cardiac device, discharge to a skilled nurse facility for more than seven days and pregnancy.
In the wearable cardioverter-defibrillator group the vest was worn on average for 14.1 hours a day, give 1.4% appropriate shocks, 0.6% inappropriate shocks and 4.6% aborted shocks.