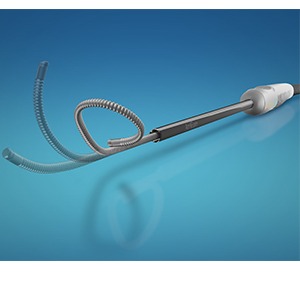
AtriCure has announced that it has received 510(k) Food and drug Administration (FDA) clearance for the cryoFORM cryoablation probe. The device, previously launched in October 2015 in the European market under a CE mark, offers increased probe flexibility to adapt to a variety of surgical ablation procedures.
The cryoFORM cryoablation probe is indicated for use in the cryosurgical treatment of cardiac arrhythmias by freezing target tissues and creating an inflammatory response that blocks the electrical conduction pathway.
According to a company release, the new probe offers increased flexibility, allowing the surgeon to more easily manipulate and apply the device and conform to challenging anatomies.
“The flexibility of cryoFORM, together with the automatic defrost function of the cryoICE system, made us decide at the Heart Center Leipzig to start using this product for our cryoablation procedures,” says Martin Misfield, professor and co-director, Department of Cardiac Surgery, Heart Center, University of Leipzig, Leipzig, Germany.












