BIBA Publishing
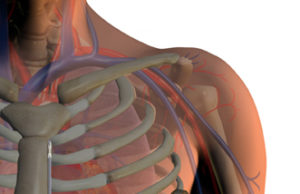
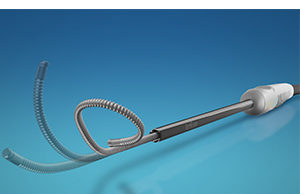
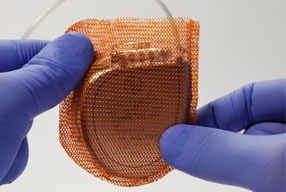
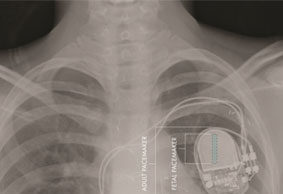
EFFORTLESS long-term study confirms safety and efficacy of Boston Scientific Subcutaneous...
Data collected from the EFFORTLESS study were presented as a late-breaking clinical trial at the 37th Annual Scientific Sessions of the Heart Rhythm Society (HRS) in San Francisco, USA.
Acutus Medical granted CE mark for AcQMap 3D
Acutus Medical has received CE mark approval for its AcQMap high resolution imaging and mapping system, and for its AcQMap catheter.
Cell Therapy grants Japan license to Daiichi Sankyo for Heartcel
Cell Therapy has granted the Japan license for its innovative cardiac regeneration medicine, Heartcel (immuno-modulatory progenitor (iMP) cells) to Daiichi Sankyo.
Large transvenous lead extraction study finds 95.7% of individual leads successfully...
The research was presented the Heart Rhythm Society's 37th Annual Scientific Sessions, and includes data from both high volume and low volume medical centres.
Studies support feasibility of extravascular implantable cardioverter defibrillation therapy
Several studies evaluating a novel approach to implantable cardioverter defibrillator (ICD) have shown the feasibility of therapy using Medtronic's EV-ICD system, according to a company release.
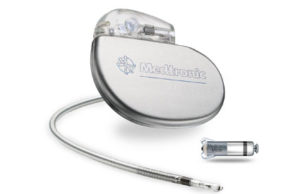
New data support safety and performance of Medtronic Micra system
Medtronic has announced clinical results highlighting the safety and performance profile of the miniaturised Micra transcatheter pacing system (TPS) at the 2016 Heart Rhythm Society meeting.
Routine ganglionic plexus ablation “should not be performed” in advanced atrial...
Results from the AFACT trial have shown no clinical benefits and significantly more complications associated with routine ganglionic plexus ablation for advanced atrial fibrillation patients. Data were presented at the 37th Heart Rhythm Society Scientific Sessions (HRS; 4-7 May, San Francisco, USA).
St Jude Medical MultiPoint Pacing Technology could achieve 87% patient response...
Results of St Jude Medical's MultiPoint Pacing investigational device exemption clinical study have been presented during a late-breaking clinical trial session at the Heart Rhythm Society's (HRS) 37th annual scientific sessions.
George Van Hare
George Van Hare (The Louis Larrick Ward professor of Pediatrics and director, Division of Pediatric Cardiology, Washington University School of Medicine, St Louis, USA), has worked towards advancing paediatric electrophysiology, as a recognised specialty, in the paediatric cardiology world and the electrophysiology world. He considers that working as a paediatric electrophysiologist is a "rewarding" experience, because it has allowed him to diagnose and cure at a very early stage. He talks to C
Merit Medical launches interventional cardiac resynchronisation therapy initiative
Merit Medical Systems has officially launch its Interventional cardiac resynchronisation therapy initiative during the Heart Rhythm Society (HRS) Meeting in San Francisco, USA.
FDA approves Iperia MR conditional cardiac resynchronisation defibrillators
The Iperia ProMRI HF-T cardiac resynchronisation defibrillator has been approved by the US Food and Drug Administration (FDA).
Stereotaxis to highlight new clinical results at HRS 2016
Stereotaxis will share results of recently published clinical studies, new technology enhancements and simulations of its computer-controlled mapping and lesion formation capabilities at HRS 2016.
FDA approves Boston Scientific navigation-enabled IntellaNav catheters
Boston Scientific has received US Food and Drug Administration (FDA) approval for two catheters that can be used with the company's Rhythmia mapping system.
Boston Scientific announces Heart Rhythm Society 2016 presentations
Boston Scientific has announced key data, including one late-breaking clinical trial, that will be featured at the 37th Annual Scientific Sessions of the Heart Rhythm Society (HRS) in San Francisco on May 4-7 2016.
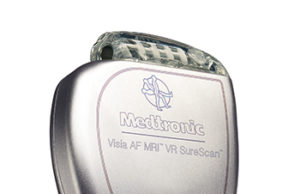
US FDA grants Medtronic approval for Visia single-chamber implantable cardioverter defibrillators
Medtronic has announced it has received US Food and Drug Administration (FDA) approval for the Visia AF MRI SureScan and Visia AF single-chamber implantable cardioverter defibrillators (ICDs).
Seventy-fifth patient enrolled in GENETIC-AF bucindolol trial
The 75th patient has been enrolled in GENETIC-AF, a phase 2B/3 clinical trial evaluating bucindolol (Gencaro, Arca Biopharma) as a potential treatment for atrial fibrillation.
Boston Scientific receives CE mark for MRI labelling of Emblem S-ICD...
Boston Scientific has received CE mark approval for the new Emblem MRI subcutaneous implantable defibrillator (S-ICD) system, as well as magnetic resonance (MR) conditional labelling for all previously implanted Emblem S-ICD systems.
Empagliflozin (Jardiance) to be studied for the treatment of people with...
Boehringer Ingelheim and Eli Lilly are to conduct two outcome trials investigating the diabetes medicine empagliflozin (Jardiance) for the treatment of people with chronic heart failure.
Medtronic’s Reveal LINQ detects atrial fibrillation at higher rate than previously...
Medtronic has announced one-year results from a real-world study of patients who had a cryptogenic stroke, or stroke of unknown cause.
St Jude Medical announces EnSite Precision cardiac mapping system limited market...
St Jude Medical has announced expansion of its EnSite Precision cardiac mapping system limited market release in Europe and use of the new platform in more than 600 cases in nine countries since receiving CE mark in January 2016.
AtriCure receives FDA clearance for its cryoFORM cryoablation probe
AtriCure has received US Food and Drug Administration 510(k) clearance for the cryoFORM cryoablation probe, which is designed to offer increased probe flexibility to adapt to a variety of surgical cardiac ablation procedures.
Biotronik gets FDA approval for its BioMonitor 2 insertable cardiac monitor
Biotronik has announced Food and Drug Administration (FDA) approval of BioMonitor 2, an insertable cardiac remote monitor with ProMRI technology.
Healthcare workers’ radiation exposure tied to range of health problems
Healthcare professionals performing x-ray guided cardiovascular procedures may be at higher risk for health problems including orthopaedic problems, cataracts, skin lesions and cancers, according to new research.
INOVATE-HF data show no evidence that vagus nerve stimulation reduces death...
Results from the INOVATE-HF trial indicate that vagus nerve stimulation does not reduce the rate of death or heart failure events in chronic heart failure patients. The data were simultaneously presented at ACC 2016 and published in the Journal of the American College of Cardiology.
First leadless pacemaker approved in the USA
Medtronic has announced it has received US Food and Drug Administration (FDA) approval for its Micra Transcatheter Pacing System (TPS). The leadless device, which is 93% smaller than conventional pacemakers, provides a safe alternative for pacing without the complications associated with cardiac leads.
Development and commercialisation of andexanet alfa in Japan fully supported by...
Portola Pharmaceuticals has announced that it has entered into a clinical collaboration agreement with Daiichi Sankyo to develop andexanet alfa as an antidote for edoxaban, Daiichi Sankyo's Factor Xa inhibitor, in Japan.
American College of Cardiology elects A Allen Seals as chair of...
A Allen Seals has been elected chair of the American College of Cardiology (ACC) Board of Governors and secretary of the Board of Trustees, the main governing body of the ACC, for 2016-2017.
Richard Chazal assumes American College of Cardiology presidency
Richard Chazal has assumed his role as president of the American College of Cardiology (ACC) during the Convocation Ceremony held in conjunction with the ACC's 65th Annual Scientific Session in Chicago.
HeartLight System gets FDA approval for atrial fibrillation treatment
CardioFocus has announced that it has received premarket approval from the US Food and Drug Administration (FDA) for its HeartLight Endoscopic Ablation System for the treatment of patients with paroxysmal atrial fibrillation.
New independent data confirms benefits of CardioMEMS HF System
Northwell Health physicians presented data at the American College of Cardiology 65th Annual Scientific Session (2-4 April, Chicago, USA) showing heart failure management with the CardioMEMS HF System leads to significant improvements in quality of life and exercise capacity for patients with heart failure.
Two studies report on missed opportunities to avoid painful shocks at...
Many patients who have an implantable cardioverter defibrillator (ICD) are unaware that the device can be deactivated to prevent painful shocks in their final days of life, according to two new studies.
Test to predict risk of sudden cardiac death being developed in...
A new test to identify patients at risk of sudden cardiac death is being developed by researchers at the University of Leicester and Leicester's Hopsitals following a £183,000 grant from national charity Heart Research UK.
4WARD Coalition launches website to empower patient/healthcare provider communication
The 4WARD Coalition has launched AFib4WARD.com, an online tool designed to help non-valvular atrial fibrillation patients and their healthcare providers engage in informed discussions and shared decision making.
Intravenous vernakalant superior to ibutilide in recent on-set AF patients
Researchers in Austria have found shorter time to sinus rhythm in patients with recent-onset atrial fibrillation (AF) treated with intravenous vernakalant (Brinavess, Cardiome Pharma) compared with ibutilide treatment.
Stereotaxis completes patient enrolment for Niobe system’s post-market surveillance in Japan
Stereotaxis has announced that Takatsuki General Hospital in Japan has reached the milestone of treating 120 patients using the company's Niobe remote magnetic navigation system. This milestone completes the required patient enrolment for the Niobe system's post-market surveillance in Japan.
Presence of a wide and large S-wave in lead I is...
New research published in the Journal of the American College of Cardiology indicates that a wide/and or large S-wave in lead I is a powerful predictor of life-threatening ventricular arrhythmias in Brugada Syndrome patients with no history of cardiac arrest at presentation.
CE mark approval granted for new St Jude Medical Quartet quadripolar...
St Jude Medical has announced the launch and CE mark approval for three new Quartet left ventricular (LV) leads.
Zoll receives approval to market Thermogard XP in Japan
Zoll Medical has announced that its Japanese subsidiary, Asahi Kasei Zoll Medical, has obtained approval from Japan's Pharmaceuticals and Medical Devices Agency (PMDA) to market the company's Thermogard XP.
AHA releases advisory proposing wearable defibrillators as potential treatment option
A wearable automatic defibrillator may be an option for patients who are at risk for life-threatening heart rhythm abnormalities but are not good candidates for an implantable cardiac defibrillator, according to an advisory for the American Heart Association (AHA).
Atrial fibrillation patients at highest stroke risk not prescribed necessary medication
Nearly half of all atrial fibrillation patients at the highest risk for stroke are not being prescribed blood thinners by their cardiologists, according to a new study.
Sir Nilesh Samani appointed as next British Heart Foundation medical director
Sir Nilesh Samani has been announced as the next medical director of the British Heart Foundation. He will succeed Peter Weissberg who will retire in October 2016.
FDA proposes ban on most powdered medical gloves
The US Food and Drug Administration has announced a proposal to ban most powdered gloves in the USA. While use of these gloves is decreasing, they pose an unreasonable and substantial risk of illness or injury, according to an FDA news release.
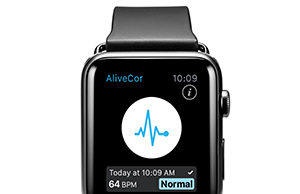
AliveCor introduces Kardia Band electrocardiogram for Apple Watch
AliveCor has introduced the first medical-grade Electrocardiogram (EKG) band for the Apple Watch, the Kardia Band, along with a new app for smartphones.
FDA grants 510(k) clearance for InfoBionic MoMe Kardia
InfoBionic has received 510(k) clearance from the US Food and Drug Administration (FDA) for MoMe Kardia, a wireless, remote monitoring system.
Arca Biopharma announces update on GENETIC-AF trial
Arca Biopharma has announced the GENETIC-AF trial, which will evaluate bucindolol (Gencaro) as a potential treatment for atrial fibrillation.
NICE publishes final recommendation for sacubitril/valsartan
The UK National Institute for Health and Care Excellence (NICE) has recommended sacubitril/valsartan (Entresto) in its final draft guidance for use within the UK National Health Service.
Fluoroscopy times and radiation dose reduced in atrial fibrillation ablation with...
A real-world study has found that the SmartTouch (Biosense Webster) contact force-sensing catheter coupled with an Advanced Catheter Location feature during atrial fibrillation (AF) ablation reduced fluoroscopy times by 77%, radiation dose by 71% and procedural time by 19%.
New bidirectional mechanical lead extraction device is safe and efficient with...
A new bidirectional rotational mechanical lead extraction sheath is both safe and effective in performing lead extraction, according to a study published online ahead-of-print in Europace. Clinical success for the leads extracted using this novel sheath was 98.1% with no mortality or major complications.
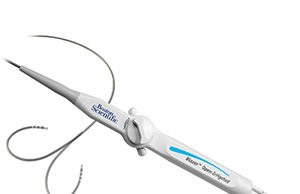
Boston Scientific receives FDA approval for Blazer open-irrigated catheter
The approval of the Blazer OI catheter marks the first time Boston Scientific will offer an open-irrigated catheter to the US market.
BioMonitor 2 released in the UK and Ireland
BioMonitor 2 (Biotronik) is now available for patients in the UK and Ireland. The insertable device is designed to allow accurate and reliable continuous detection of cardiac electrical events.
Zoll announces several management changes
Zoll Medical has announced a new chief executive officer and senior vice president of resuscitation as Richard A Packer is to lead Asahi Kasei's Healthcare Business Unit.
St Jude Medical granted CE mark approval for MRI compatible labelling...
CE mark approval has been secured for the magnetic resonance (MR) conditional labelling for 1.5T scans for the Nanostim leadless pacemaker from St Jude Medical.
First implantations of EBR Systems’ WiSE technology take place
The first commercial implantations of WiSE (wireless stimulation endocardially) technology (EBR Systems) have taken place in the UK and Czech Republic.
Hypertrophic cardiomyopathy patients at risk of sudden cardiac death may be...
Patients with hypertrophic cardiomyopathy at risk of sudden cardiac death and without pacing indication may be eligible for Subcutaneous Implantable Cardioverter Defibrillator (S-ICD) implantation, according to results of a single-centre study.
APN Health granted FDA clearance for Navid 3D cardiac mapping system
The system produces real-time 3D catheter location information from 2D fluoroscopic images of the heart, and correlates them with the electrical activation of the heart.
Biotronik opens training facility and innovation centre in New York City,...
Biotronik has opened the Education and Innovation Center in New York, USA to hold educational programmes.
Boston Scientific receives FDA approval for Acuity X4 quadripolar leads
The US Food and Drug Administration has granted approval to Boston Scientific for its Acuity X4 quadripolar left ventricular leads. The company can now offer its first full X4 cardiac resynchronisation system to the US market.
Medtronic receives CE mark for three MRI-compatible cardiac resynchronisation therapy defibrillators
The three cardiac resynchronisation therapy defibrillators (CRT-Ds) are approved for 3 Tesla magnetic resonance imaging scans. These devices are the first and only CRT-Ds approved for this level of MRI, according to a company release.
American College of Cardiology releases competency guidelines for general cardiologists
The American College of Cardiology has release its 2016 ACC Lifelong Learning Competencies for General Cardiologists. This document defines the knowledge, skills and behaviours expected of practising clinical cardiologists.
American Heart Association warns that African Americans and Hispanics face greater...
Studies have shown that heart failure affects African American individuals with roughly twice the incidence of that of Caucasians. The Hispanic population has the second-highest risk of developing heart failure in the USA.
FDA panel offers guidance on leadless pacing technology
The panel provided insight around patient selection and post approval study methodology, folowing a panel discussion on leadless pacing technology.
FDA Panel provides recommendations on adverse events, long-term safety and postmarket...
The FDA's Circulatory System Devices Panel of the Medical Devices Advisory has made recommendations for leadless pacemakers regarding adverse events, long-term safety issues (including battery longevity), necessary elements for postmarket surveillance, indications for use and labelling, and implanting physicians' training.
St Jude Medical receives FDA approval for MultiPoint pacing technology
This technology is designed to provide additional options which may benefit cardiac resynchronisation therapy patients who are not responsive to other methods of pacing.
First patient enrolled in AtriCure postoperative atrial fibrillation study
The first patient has been enrolled in the ATLAS (AtriClip Left Atrial Appendage Exclusion Concomitant to Structural Heart Procedures) clinical study.
Idarucizumab reimbursement granted in England, Ireland and Wales
The agreement of the English National Institute for Health and Care Excellence, the All Wales Medicines Strategy Group and the Irish National Centre for Pharmoeconomics has granted the drug eligibility for full reimbursement, without the need for a full appraisal in these countries.
Left atrial appendage isolation with cryoballoon may work as an adjunctive...
Researchers in Turkey have found that additional left atrial appendage (LAA) isolation using second generation cryoballoon technology is feasible and safe and that it may be considered as an adjunctive therapy to pulmonary vein isolation for persistent atrial fibrillation (AF) treatment.
Regenerate Life Science formed to develop advancement of cardiovascular medicine with...
The company will concentrate on developing new technologies to aid in the advancement of cardiovascular medicines using stem cell therapy.
Robotically-guided approach offers alternative LV lead implantation through the coronary sinus
Left ventricular (LV) lead implantation for cardiac resynchronisation therapy (CRT) with a robotically-guided surgical approach through the coronary sinus seems to offer a new alternative when conventional approaches are not suitable, a new study has found.
Biotronik Itrevia 7 HF-T resynchronisation therapy defibrillator approved in Japan for...
The Japanese launch of the device took place in July 2015. This is the first and only CRT-D in Japan with such conditions, according to a company release. All already implanted devices have been deemed safe for full-body MRI scans at 1.5 tesla strength.
FDA announces Advisory Committee meeting on leadless pacing
Members of the FDA's Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet on 18 February 2016 to provide advice and recommendations on leadless cardiac pacemaker device technology.
Spectranetics Bridge occlusion balloon cleared by the US Food and Drug...
The company has received premarket notification 510(k) clearance for the Bridge Occlusion Balloon for lead extraction procedures. This clearance will initiate a controlled market release, according to a Spectranetics release, with full market launch at the Heart Rhythm Society's 37th Annual Scientific Sessions.
Implantation of multiple leadless pacemakers is feasible without impacting cardiac function
An animal study from China has indicated that it is feasible to implant two leadless pacemakers in the right ventricle of the same heart without impacting cardiac function at six months.
New drug candidate may prevent and reverse hypertrophic cardiomyopathy in mouse...
MYK-461, a drug candidate from MyoKardia, may prevent and reverse development of hypertrophic cardiomyopathy in multiple genetic mouse models, according to a study published in Science.
Medtronic first to receive FDA approval for MR-conditional CRT-Ds
Medtronic has announced that it is the first company to receive US Food and Drug Administration approval for magnetic resonance imaging (MRI) conditional cardiac resynchronisation therapy (CRT) defibrillators for the treatment of heart failure.
Cardiologists expose concerns regarding battery life of cardiac implantable electronic devices
The battery life of cardiac implantable electronic devices must be improved to reduce the need for replacement and the risks this carries for patients, argue UK cardiologists John Dean and Neil Sulke in an editorial published in BMJ.
Abbott acquires Kalila Medical
Kalila Medical has a novel steerable sheath that is designed to help physicians more easily access and perform catheter-based electrophysiology procedures.
Portola license Japanese commercial rights for andexanet alfa to Bristol-Myers Squibb...
Portola has also entered into a clinical collaboration agreement with Bayer HealthCare to include its Factor Xa inhibitor rivaroxaban in this clinical development programme in Japan.
Biotronik receives CE mark for new Ilivia implantable cardioverter defibrillators and...
Ilivia devices come with the company's ProMRI technology, as well as MRI AutoDetect, which is designed to allow the cardiologist to activate a window in which all device functionality is maintained until a patient actually undergoes an MRI scan.
Arca enrols 50th patient in GENETIC-AF atrial fibrillation prevention trial
This trial evaluates bucindolol hydrochloride (Gencaro) as a potential genetically-targeted treatment for the prevention of atrial fibrillation.
Stereotaxis and Philips extend development collaboration for Niobe ES and Allura...
Stereotaxis and Philips have signed an addendum, pursuant to their existing development and cooperation agreement, to facilitate development of a new interface between each company's most advanced systems for electrophysiology and interventional cardiology procedures.
Optisure high voltage leads classified as Class 1 Advisory by US...
A previously communicated voluntary global field safety action related to St Jude Medical's Optisure Dual Coil Defibrillation Leads has now been classified as a Class 1 Advisory by the US Food and Drug Administration.
German public health body grant once-daily edoxaban indication for prevention of...
The German Federal Joint Committee (Gemeinsamer Bundesausschuss-G-BA) has granted Daiichi Sankyo's edoxaban (Lixiana) an indication of a minor additional benefit.
AtriCure receives approval for AtriClip products from Japanese Ministry of Health,...
AtriCure has received approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for its AtriClip products, which will be distributed in Japan by Century Medical.
Sacubitril/valsartan now available in the UK for the treatment of HFrEF...
Sacubitril/valsartan (Entresto, Novartis) has been made available in the UK for the treatment of adult patients with symptomatic chronic heart failure with reduced ejection fraction.
Imricor Medical Systems’ Advantage-MR EP recorder and stimulator system granted CE...
The Advantage-MR EP recorder/stimulator system is a magnetic resonance conditional recording system for magnetic resonance imaging-guided electrophysiology procedures.
Women and men with heart failure benefit similarly from implantable cardioverter...
An analysis from the US Get with the guidelines for heart failure registry has found that women and men with heart failure and reduced left ventricular ejection fraction benefit similarly from implantable cardioverter defibrillators.
Jonathan Kalman
A leading heart rhythm expert in Australia, Jonathan M Kalman directs both clinical and research groups in the Department of Cardiac Arrhythmias at the Royal Melbourne Hospital and University of Melbourne, Australia. In this interview, he recalls details of the first curative ablation procedure for atrial fibrillation in Australia and talks about the impact of lifestyle modification in atrial fibrillation management.
Has leadless pacing changed practice?
Chu-Pak Lau explores the development and use of externally-powered and entirely intracardiac leadless pacemakers, and considers the effect they have had on clinical practice.
Verseon presents data on new class of anticoagulants at the 8th...
The recently-obtained data demonstrates continuing and steady progress for the drug candidates in Verseon's anticoagulation program, according to a press release.
AliveCor partners with LifeWatch to expand into remote patient monitoring
AliveCor are to partner with LifeWatch AG. AliveCor Mobile ECG will be integrated into LifeWatch's remote cardiac monitoring service. This represents AliveCor's first step into remote patient monitoring.
Stereotaxis begins multicentre, randomised superiority study on ventricular tachycardia ablation outcomes
Stereotaxis has initiated its first prospective, multicentre, randomised clinical study to compare radiofrequency ablation outcomes generated using its Niobe ES remote magnetic navigation system to manual approaches in ischaemic scar ventricular tachycardia (VT) patients.
In the absence of testing, ICD optimisation should always be done
Mark W Kroll (Minneapolis, USA) writes about the importance of optimisation of implantable cardioverter defibrillators (ICDs) in the absence of defibrillation threshold testing.
New ultrasound-based system shows promise mapping atrial fibrillation
Initial findings have shown that a new ultrasound-based imaging system with continuous dipole density mapping provides real-time rapid global left atrial reconstruction and compares favourably to segmented computed tomography (CT).
Using the American Heart Association’s seven measures for heart health may...
Scoring highly on the American Heart Association (AHA)'s Life's Simple 7 checklist has been associated with a reduction in heart failure risk, according to a study published Circulation: Heart Failure.
Biotronik Iperia ICD systems with ProMRI technology receive FDA approval
Biotronik has been granted US Food and Drug Administration approval for use of a group of implantable cardioverter defibrillator systems with magnetic resonance imaging scans.
Biologics License Application submission completed for andexanet alfa
Portola Pharmaceuticals have completed the submission of a Biologics License Application for its investigational agent andexanet alfa to the US Food and Drug Administration.
LifeWatch Services acquires INR monitoring company, FlexLife Health
LifeWatch Services has acquired FlexLife Health, a company which offers patients remote services to monitor and measure coagulation.
Zoll LiveVest wearable defibrillator receives FDA approval for paediatric use
LiveVest is now approved for use by certain children who are at risk for sudden cardiac arrest, but who are not candidates for an implantable defibrillator device
LuxCath technology demonstrates promising results during first-in-man cardiac ablation procedures
The LuxCath optical tissue interrogation technology was used in eleven patients suffering from arrhythmias such as atrial flutter, AV nodal re-entrant tachycardia, and atrial fibrillation.
American Heart Association update shows that one in every three deaths...
This update-which has been produced since 1958-is made up from the most-recent data available compiled by the AHA, the National Institutes of Health, the Centers for Disease Control and Prevention and other government sources.
Advances in left atrial appendage occlusion and peri-procedural challenges
Dhiraj Gupta (Liverpool, UK) overviews developments in two left atrial appendage (LAA) occlusion devices, which have helped make the implant procedure safer, easier and quicker. He also highlights current challenges related to overall peri-procedural patient management.
First patient enrolled in international CEASE atrial fibrillation study
The first patient has been enrolled in the CEASE (combined endoscopic epicardial and percutaneous) atrial fibrillation (AF) clinical study, according to a press release from AtriCure.
New study evaluating whether pacemakers with closed loop stimulation prevent reflex...
BIOSync CLS will investigate the efficacy of the uniquely physiologic rate response sensor CLS in preventing syncope.
University of Washington to lead US expansion of Medtronic’s HeartRescue project
HeartRescue is a Medtronic Philanthropy partnership launched in five US states in 2010, which aims to improve Sudden Cardiac Arrest (SCA) survival rates.
Industry’s first WiFi-based remote cardiac rhythm monitor introduced by ScottCare Corporation
The device enables the clinician to access real-time streaming for live patient visibility, auto-detection of arrhythmia events, and wireless transmission of three channels of ECG data.
Bridge occlusion balloon submitted for FDA 510(k) Premarket Notification for lead...
The device from Spectranetics is designed to substantially reduce blood loss in the event of a superior vena cava tear.
Biotronik and cardiology experts join Heartbeat International for scientific symposium
Biotronik is to support its charity partner, Heartbeat International (HBI) at the 25th Interamerican Congress of Cardiology, in Santiago, Chile.
Patients with chronic kidney disease benefit from pacing left and right...
Cardiac resynchronisation therapy with defibrillator may prevent hospitalisation due to heart failure, when compared to treatment with implantable cardioverter defibrillator alone.
Insertable cardiac monitors may help decide safe discontinuation of oral anticoagulation...
A strategy including insertable cardiac monitors (ICM) to guide rhythm control with antiarrhythmic drugs and assessment of AF burden may allow safe discontinuation of oral anticoagulation in AF patients at high risk of bleeding.
Biotronik releases Eluna 8 pacemaker in Japan
The device is approved for use during 1.5 Tesla full-body MRI scans and ultra-high strength 3.0 Tesla MRI scans with an exclusion zone.
Leading heart failure doctors pledge united action to improve patient outcomes
Leading heart failure doctors from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) have united in a pledge to improve patient outcomes and reduce the burden of heart failure on society.
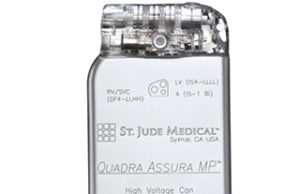
St Jude Medical gets CE mark for MRI compatibility on Quadra...
The Quadra Assura cardiac resynchronisation therapy defibrillator (CRT-D) is now approved for use with magnetic resonance imaging (MRI) scanning systems at 1.5 Tesla.
B3 trial to evaluate benefits of unique rate response algorithm for...
This trial aims to determine if closed loop stimulation can delay the onset of atrial fibrillation and reduce the risk of stroke.
Medtronic recalls three models of pacemakers due to battery impedance
Medtronic has reported an issue with the long-term battery performance of its InSync III cardiac resynchronisation therapy-pacemakers (CRT-P) (models 8042, 8042B and 8042U). The Food and Drug Administration (FDA) has designated this issue as a Class II recall.
Idarucizumab licensed in Europe as specific reversal agent of dabigatran
Boehringer Ingelheim has announced that the European Commission has licensed idarucizumab (Praxbind) for rapid and specific reversal of the anticoagulant effects of dabigatran etexilate in cases of emergency surgery/urgent procedures or in situations of life-threatening or uncontrolled bleeding.
Adults with congenital heart defects at considerably higher risk of stroke
A study has shown heart failure, diabetes and recent heart attacks to be the strongest predictors of stroke caused by a blocked artery.
Four societies release collaborative expert consensus statement on ICD programming and...
These global recommendations are from The Heart Rhythm Society (HRS), European Heart Rhythm Association (EHRA), Asia Pacific Heart Rhythm Society (APHRS), and the Socieded Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE).
Novartis’ Entresto receives European authorisation for chronic heart failure treatment
Novartis' Entresto (sacubitril/valsartan) has been authorised for the treatment of adult patients with symptomatic chronic heart failure with reduced ejection fraction (HFrEF).
Portola Pharmaceuticals’ Phase 3 ANNEXATM study results published
The results were published online by The New England Journal of Medicine, while the ANNEXA-R data were presented during a late-breaking clinical trial session at AHA Scientific Sessions 2015.
Ebit receives 2015 Frost and Sullivan European Award for Technology Leadership
This award recognises Ebit's Suitestensa cardiovascular information system (CVIS) integration platform.
Biosense Webster acquires Coherex Medical
This acquisition adds the Coherex WaveCrest left atrial appendage (LAA) occlusion system to BioSense's portfolio.
Micra pacemaker from Medtronic wins 2015 ‘Best of What’s New’ award...
The Micra pacemaker was chosen from thousands of submissions, and is one of among 100 honourees that are seen represent a significant leap forward in their respective categories by the magazine.
Greater long-term success rates for Stereotaxis’ Niobe system compared to manual...
The results of an independent, multicentre study, which looked at the procedural benefits and outcomes of patients undergoing radio frequency ablation therapy for ventricular tachycardia, were published at the AHA.
Medtronic launch newly-FDA approved MyCareLink Smart app-based monitor
According to Medtronic, this is the world's first app-based remote monitoring system for patients with implantable pacemakers.
BioMonitor 2 cardiac remote monitor launched in Europe by Biotronik
The new device is a subcutaneous, insertable cardiac remote monitor designed to continuously monitor
cardiac electrical events reliably and accurately.
New study has “major physiopathological and clinical implications” for the management...
Josep Brugada (Barcelona, Spain), Carlo Pappone (Milan, Italy) and others report in Circulation: Arrhythmia and Electrophysiology that ablation of abnormal epicardial substrate in patients with Brugada syndrome can eliminate the phenotype expression of the syndrome.
AliveCor appoints Vic Gundotra as new chief executive officer
Vic Gundotra has worked as a senior vice president at Google, after spending 15 years with Microsoft.
Micra TPS meets global clinical trial safety and effectiveness endpoints
The transcatheter pacing system from Medtronic is the smallest pacemaker on the market.
Partners of heart defibrillator patients more concerned about resuming sex
Concerns for both patients and their partners declined after three months.
Global symposium on Advanced Concepts in Electrophysiology receives support from Biotronik
The symposium is an educational program for electrophysiologists and device-oriented cardiologists.
Study shows increased risk of cardiac arrhythmias and cardiac death with...
The meta-analysis-which covered 33 studies involving more than 20 million patients-has been published in the Journal of the American College of Cardiology.
Debate on defibrillation testing continues
Speaking at a debate about the value of defibrillator threshold testing in patients with ICDs, Klaus Witte (Leeds, UK) told delegates at the Heart Rhythm Congress that such testing was a "pointless" intervention because it does not provide benefit and may be associated with adverse events. Nick Linker (Middlesbrough, UK), who argued against Witte, said that there is still a significant proportion of patients-who were not included in the major trials of defibrillation testing-for whom
LivaNova announces launch of the Platinum ICD & CRT-D range
Platinum is designed to protect patients from avoidable replacement surgeries and the inherent risk of complications.
New recommendations green-light some athletes with heart disease to compete in...
The recommendations from the American Heart Association and the American College of Cardiology may permit participation in competitive sports for some athletes with long QT syndrome
Psychological implications and management for patients with implantable cardioverter defibrillators
The implantation of an implantable cardioverter defibrillator (ICD) device poses numerous psychosocial challenges, which have been shown to significantly influence functional outcomes in this population. Therefore, the optimisation of medical therapy and provision of psychological support is key to managing the patient's biopsychosocial functioning, write Elizabeth Banwell, Katie Murray and Stephen Gunning.
Blackouts and near drownings may signal sudden death risk
Blackouts and near drownings may point to long QT syndrome (LQTS), signalling an increased risk of sudden death, according to research presented at the SA Heart Congress (25-28 October, Sun City, South Africa) by Paul Brink, Tygerberg, South Africa.
Medtronic receives CE mark for new single-chamber ICDs designed to detect...
The Visia AF and Visia AF MRI SureScan are designed to detect and monitor new onset, asymptomatic and previously undiagnosed atrial fibrillation.
Idarucizumab gets accelerated FDA approval for use in patients in need...
Idarucizumab (Praxbind) is the first reversal agent approved specifically for dabigatran and works by binding to the drug compound to neutralise its effect.
Pacemakers identify atrial fibrillation and enable initiation of stroke prevention
According to research presented at Acute Cardiovascular Care 2015, pacemaker checks are a good way to identify new cases of atrial fibrillation so that anticoagulation can be started to prevent strokes.
The American Heart Association and Heart Rhythm Society coordinate to improve...
The American Heart Association (AHA) and Heart Rhythm Society (HRS) have announced a collaboration designed to improve the quality of care of atrial fibrillation (AFib) patients and advance to cardiovascular research.
Michael Glikson
Michael Glikson (Tel Hashomer, Israel) has contributed to the development of technologies for CRT, modern lead extraction, advanced mapping ablation of AF and VT and LAA occlusion. Glikson's current projects as president of the Israel Heart Society and co-president of the International Dead Sea Symposium (IDSS) on Innovations in Cardiac Arrhythmias and Device Therapy reflect his innovative approach in the field. He speaks to Cardiac Rhythm News about these projects, other highlights in his
CardioKinetix announces South Korean approval for the Parachute System for heart...
Regulatory approval has been granted in South Korea for the Parachute System by CardioKinetix by the Korean Ministry of Food and Drug Safety (MFDS).
St Jude Medical completes acquisition of Thoratec
St Jude Medical has announced its completion of the previously announced acquisition of Thoratec Corporation, a global leader in mechanical circulatory support technology for the treatment of advanced heart failure.
St Jude Medical announces CE mark approval for the HeartMate 3...
St Jude Medical has announced that it has received CE mark approval for the HeartMate 3 Left Ventricular Assist System (LVAS). This is a cardiac support option for advanced heart failure patients awaiting transplantation who are not candidates for heart transplantation, or in myocardial recovery.
Role of rotors in mapping and ablation of ventricular fibrillation
In recently published studies, modulation of rotors by ablation seems to be effective in termination of atrial fibrillation. Now, researchers are exploring the role of rotors in ventricular fibrillation. Siva K Mulpuru (Mayo Clinic, Rochester, USA), discusses the latest research in this field.
AtriCure to acquire nContact
AtriCure has entered into a definitive merger agreement under which it will acquire nContact, a privately-held developer of innovative cardiac ablation solutions.
First wireless cardiac pacing system for heart failure receives the CE...
EBR Systems has announced the CE mark approval for its Wise (Wireless stimulation endocardially) technology, which is the world's only wireless endocardial pacing system for cardiac resynchronisation therapy (CRT).
AtriCure announces launch of the Cryoform cryoablation probe
Atricure has announced the launch of the Cryoform cryoablation probe, which offers increased probe flexibility to adapt to a variety of surgical ablation procedures.
Acutus Medical expands leadership team
John Dahldorf, Martin Chambers and Steven McQuillan join the company as chief financial officer, chief commercial officer and senior vice president, Regulatory and Clinical Affairs, respectively.
Benefits of autonomic regulation therapy for heart failure are maintained after...
Data show that autonomic regulation therapy in patients with moderate to severe chronic heart failure and impaired heart function is well tolerated, safe, improves the heart's ability to pump blood, and reduces the frequency and severity of symptoms associated with chronic heart failure.
Novel oral anticoagulants require improved safety data to challenge warfarin
More real-world safety data could potentially address the issue of under-dosing and under-treatment with novel oral anticoagulants (NOACs) in global practice, says an analyst with research and consulting firm GlobalData.
European Medicines Agency recommends dabigatran antidote after accelerated assessment
The European Medicines Agency (EMA) has recommended granting a marketing authorisation, following accelerated assessment, for idarucizumab (Praxbind) as a specific antidote to dabigatran etexilate (Pradaxa).
Entresto (sacubitril valsartan) recommended by CHMP for EU approval
Pending final approval by the European Commission, Entresto (sacubitril valsartan) will be licensed for use in the UK for the treatment of adult patients with symptomatic chronic heart failure and reduced ejection fraction.
NICE recommends once-daily Lixiana (edoxaban) for preventing stroke and systemic embolism...
The National Institute for Health and Care Excellence (NICE), the medicines cost-effectiveness body for England and Wales, has recommended a new treatment to help prevent stroke and systemic embolism in patients suffering from atrial fibrillation.
Societies release new guideline for treating supraventricular tachycardia
A new guideline aimed at helping clinicians treat patients with supraventricular tachycardia has been released by the American College of Cardiology, American Heart Association, and Heart Rhythm Society.
US societies release updated training requirements for clinical electrophysiology
Due to the complex nature of clinical cardiac electrophysiology, an updated training statement released by the American College of Cardiology, the American Heart Association and the Heart Rhythm Society, is calling for increased training for practitioners.
Laguna Pharmaceuticals initiates phase 3 study of vanoxerine in atrial fibrillation...
The study, known as RESTORE SR, will enrol 600 subjects at trial sites in the USA and other countries and will evaluate vanoxerine at a 400mg dose.
New data demonstrate sustained reversal of anticoagulant effect of Factor Xa...
The second part of Portola Pharmaceuticals' phase 3 ANNEXATM-R study achieved all primary and secondary endpoints with high statistical significance.
Physio-Control to acquire HeartSine
Financial details of the transaction are not being released. The combination creates one of the world's largest AED solutions providers.
CardioFocus’ HeartLight PMA for the treatment of atrial fibrillation filed and...
The accepted PMA application includes safety and effectiveness data from the company's multicentre HeartLight pivotal clinical trial, a randomised, controlled study in which a total of 353 patients were treated at 19 US centres.
First MRI-conditional implantable cardioverter defibrillator system receives FDA approval
Medtronic has announced that it has received the first US Food and Drug Administration (FDA) approval for an implantable cardioverter defibrillator (ICD) system for use with magnetic resonance imaging (MRI) scans.
First patient enrolled in study of Eliquis (apixaban) in NVAF patients...
This two-by-two factorial, randomised controlled trial will evaluate the safety of Eliquis versus warfarin or other vitamin K antagonists (VKA) in patients with NVAF and a recent acute coronary syndrome or undergoing PCI.
Simple test predicts obstructive sleep apnoea in patients hospitalised for heart...
Researchers at Thomas Jefferson University, Philadelphia, USA, have showed that a simple questionnaire, evaluation and pulse-oximetry monitoring can lead to early detection of sleep apnoea in patients hospitalised for congestive heart failure.
Electrical left atrial appendage isolation helps to improve freedom from long-standing...
A randomised, multicentre study has found improvement in the long-term freedom from long-standing persistent atrial fibrillation (AF) in patients who underwent an ablation plus empirical electrical left atrial appendage isolation strategy. No major complications were reported.
LCZ696 to be available to UK’s NHS under the Early Access...
LCZ696 (sacubitril valsartan) will be made available to eligible patients in the UK before a final European licensing decision is made.
New real-world study reveals low bleeding rates with rivaroxaban in atrial...
Results from the real-world study XANTUS have shown low rates of major bleeding in patients with atrial fibrillation taking rivaroxaban (Xarelto, Bayer Healthcare) for stroke prevention. Data were consistent with findings from the pivotal phase III clinical trial, ROCKET-AF.
Data from five US real-world trials comparing apixaban with other oral...
The studies compared the risk of different bleeding related outcomes, including major bleeding and/or any bleeding, hospitalisation and bleeding-related 30-day readmissions in routine clinical practice setting for apixaban versus warfarin, rivaroxaban and dabigatran.
ESC recommends subcutaneous defibrillators for management of ventricular arrhythmias and prevention...
The new guidelines now recommend that subcutaneous defibrillators (S-ICDs) should be considered as an alternative to transvenous defibrillators in patients with an indication for an ICD when pacing therapy is not needed.
Global registry presents two-year outcomes data in more than 17,000 newly-diagnosed...
These two-year prospective outcomes from cohorts 1 and 2 show a mortality rate of 3.83% per person year compared to stroke rates of 1.25% per person year and major bleeding rates of 0.7% per person year.
New studies identify gene associated with sudden cardiac death
First-of-its-kind findings from two independent studies have identified a gene associated with sudden cardiac death.
Secondhand smoke during pregnancy and early childhood linked to atrial fibrillation...
A first-of-its-kind study indicates that gestational and early life secondhand smoke exposure may double one's chance of developing atrial fibrillation as an adult.
Personal MedSystems introduces CardioSecur 22-lead mobile ECG at ESC Congress 2015
The next generation of electrocardiogram (ESC) solutions can be run with a smartphone or tablet PC, four electrodes and the CardioSecur App enabling the diagnosis and localisation of cardiac ischaemia, rhythm disorders and posterior wall infarctions.
LEADLESS II shows positive safety and efficacy outcomes with Nanostim
Primary results from the LEADLESS II study have shown positive efficacy and safety outcomes with the Nanostim leadless pacemaker (St Jude Medical) for patients who require a single-chamber ventricular pacemaker.
Study highlights gender-related differences in athletes’ hearts
The study about gender differences in athletes' hearts highlights the importance of understanding how women's hearts work, and that what looks normal in men could reveal problems in women.
Sudden cardiac death in sport may occur in healthy hearts
Study reveals how physical exertion may trigger sudden death in people with apparently healthy hearts.
ESC and Boehringer Ingelheim launch new drive for innovation in cardiovascular...
The ESC Grants for Medical Research Innovation programme will see four grants of up to €400,000 each awarded to researchers or clinicians following live presentations by selected finalists to a panel of high profile experts appointed by the ESC.
Westmead Hospital provides data supporting ablation effectiveness of Stereotaxis magnetic navigation
Stereotaxis and Westmead Hospital in Australia have announced findings of a study comparing the stability of a Niobe remote magnetic navigation system catheter group and a manually controlled catheter group in a validated cardiac wall motion simulator.
BioControl Medical completes enrolment in the INOVATE-HF study of the CardioFit...
INOVATE-HF has enrolled a total of 725 patients at 86 centres in the USA and Europe, making it the largest prospective, randomised device study to evaluate the treatment of heart failure with vagus nerve stimulation.
Biotronik gets CE mark approval for last two generations of ProMRI...
Biotronik has announced CE mark approval for its last two generations of ProMRI cardiac resynchronisation therapy defibrillator (CRT-Ds) systems. The approval allows full-body MRI scanning.
Biotronik announces CE mark approval and pilot study results for BioMonitor...
Biotronik has announced CE mark approval and the results of a pilot study into the performance of the subcutaneous, insertable cardiac monitor BioMonitor 2.
Second generation cryoballoon shows favourable outcomes treating persistent atrial fibrillation
The largest study to date exploring the mid-term efficacy of cryoablation with the second generation device has shown favourable outcomes in patients with persistent atrial fibrillation. At a median follow-up of 10 months, 67% of patients were in sinus rhythm.
Boston Scientific announces CE mark for MRI compatible labelling for ICD...
This revised labelling ensures that future patients and those already implanted with these systems are able to undergo MRI scans if indicated.
E-health vital in battle against heart disease say European cardiology leaders
A European Society of Cardiology (ESC) position paper published in European Heart Journal outlines how the ESC will exploit e-health in education and research, while tackling issues of quality control and data security.
WomenHeart launches first virtual support network for women living with atrial...
The programme, which starts in September during Atrial Fibrillation Awareness Month, aims to reach women living with heart disease who do not otherwise have access to patient support services.
GARFIELD-AF Registry data to be featured at ESC Congress 2015
A satellite symposium and four GARFIELD-AF presentations will demonstrate how antithrombotic treatment patterns are evolving in the real-world and the impact on clinical outcomes in newly diagnosed atrial fibrillation patients.
Mobile technology may help people improve health behaviours
Smartphone applications and wearable sensors have the potential to help people make healthier lifestyle choices, but, according to the American Heart Association, scientific evidence of mobile health technologies' effectiveness for reducing risk factors for heart disease and stroke is limited.
Moderate physical activity associated with lower risk of heart failure in...
A new study shows that walking and cycling for 20 minutes per day has greatest impact on lowering the risk of heart failure.
Guideline-based algorithm helps to reduce syncope recurrence in old patients with...
A sequential algorithm, which includes carotid sinus massage, tilt testing and implantable loop recorder implantation, helps to reduce syncope recurrence to 9% at one year in old patients with severe recurrent syncopes, according to results from the Syncope unit project 2 (SUP 2) study.
Study highlights advantages of Stereotaxis platform in treating ventricular tachycardia
A seven-year study indicates the Stereotaxis remote magnetic navigation platform's success in ventricular tachycardia ablations compared to both contact force sensing and other manual catheters.
NICE recommends once-daily edoxaban for preventing stroke and systemic embolism in...
NICE Final Appraisal Determination recommends that edoxaban is a cost-effective use of NHS resources.
Biotronik enrols first patients in the BIO|GUARD-MI study
Study evaluates whether early detection of cardiac arrhythmias by BioMonitor with home monitoring reduces major cardiovascular events in post-acute myocardial infarction patients.
Addressing social factors critical for continued fight against heart disease and...
Deaths from heart attacks, strokes and other heart diseases have been declining, but social factors, including race, income, environment and education could reverse that trend.
Biotronik begins first study to assess relevance of defibrillator back-up after...
BioCONTINUE is the first study to investigate the relevance of defibrillator back-up following first device replacement in a heart failure patient population with a primary indication for a cardiac resynchronisation therapy defibrillator (CRT-D).
Boston Scientific to become significant shareholder in Preventice Solutions
Boston Scientific is also to become the exclusive global sales and marketing representative for the company's cardiology-related offerings.
Sensor-based electromagnetic tracking system helps to improve resynchronisation therapy implant procedures
A sensor-based electromagnetic tracking system helps to improve cardiac resynchronisation therapy (CRT) implantation facilitating speed of the procedure, reducing exposure to radiation and improving success rate of access to the coronary sinus, according to a study presented at EHRA EUROPACE - CARDIOSTIM (21-24 June, Milan, Italy).
LuxCath LLC awarded US patent for real-time lesion visualisation technology for...
The LuxCath LLC system determines electrode-tissue contact as well as monitors lesion progression during ablation and provides real-time lesion visualisation without pressure sensors or ultrasound.
Biotronik single- and dual-chamber pacemakers and ICDs approved for use in...
A press release states that the company is now the only one offering both implantable cardioverter-defibrillators (ICDs) and pacemakers approved for 3T scans.
Cardiome partner Eddingpharm to initiate Brinavess phase I study in China
The study will be conducted in healthy volunteers. If Brinavess successfully completes phase 1, Eddingpharm anticipates initiating a pivotal phase 3 study by year end.
ESC announces hot line sessions highlights
Key topics from the hot line sessions of ESC 2015 include atrial fibrillation, pacing, acute myocardial infarction, heart failure, hypertension, diabetes mellitus, pharmacology and coronary artery disease.
Biotronik announces first implantation of Itervia HF-T QP devices
Itrevia HF-T QP includes the Closed Loop Stimulation (CLS) algorithm, capable of adapting heart rate in response to physiological demands independent of body movements or respiratory rate.
Black Americans are at greater risk for sudden cardiac arrest than...
Black Americans are more likely to experience sudden cardiac arrest and at a much earlier age than their white compatriots, according to research published in Circulation.
Off-label use of the Lariat device for left atrial appendage exclusion...
A Food and Drug Administration (FDA) announcement and a study in JAMA Internal Medicine about use of the Lariat suture delivery device (SentreHeart) for left atrial appendage (LAA) closure have raised questions regarding using the device in this off-label indication.
Highlights EHRA EUROPACE-CARDIOSTIM 2015: Interview with Michael Glikson
Michael Glikson (director of Davidai Arrhythmia Center, Heart Center, Sheba Medical Center, Tel Hashomer, Israel), vice-chairperson scientific programme for EHRA EUROPACE-CARDIOSTIM 2015, talks to Cardiac Rhythm News about the highlights of this years' congress.
Air pollution from wildfires may increase risk for cardiac arrest
Exposure to fine particle air pollution during wildfires may increase risk for cardiac arrest and other acute heart problems, particularly in the elderly, a new study has found.
More than half of breast cancer patients develop diastolic dysfunction after...
A new study reports that within 12 months of completing anthracycline treatment, 57% of breast cancer patients had changes on their echocardiograms consistent with diastolic dysfunction.
FDA reports adverse events with the Lariat device for left atrial...
The US Food and Drug Administration has issued a safety communication to healthcare providers reporting deaths and serious adverse events with the use of the Lariat Suture Delivery Device (SentreHeart) and its associated devices used for left atrial appendage closure.
Hansen Medical announces enrolment of initial cohort in ARTISAN US clinical...
The trial will evaluate the safety and effectiveness of the Sensei robotic system and Artisan family of catheters for introducing and positioning radiofrequency ablation catheters in patients with symptomatic, drug-refractory paroxysmal atrial fibrillation.
LCZ696 (Entresto) gets FDA approval for heart failure treatment
Novartis has announced that the US Food and Drug Administration (FDA) has approved Entresto (sacubitril/valsartan) tablets, previously known as LCZ696, for the treatment of heart failure with reduced ejection fraction.
Racial and gender disparities in care exist for patients newly-diagnosed with...
New study published in HeartRhythm shows differences in the utilisation of atrial fibrillation therapies in a large nationwide population.
Biotronik announces start of BIOWOMEN study
First patient enrolled in global clinical investigation into differences in gender response to cardiac resynchronisation therapy.
New research outlines more effective diagnosis for people with heart conditions
Michael Gallimore, from the School of Engineering at the University of Lincoln, UK, and colleagues have created a new algorithm which produces more accurate electrocardiogram (ECG) signal classification when tested on patients.
Less than 10% of eligible older patients receive an ICD after...
Sean D Pokorney (Durham, USA) and others report in JAMA that only 8.1% of older patients who are eligible to receive an implantable cardioverter defibrillator (ICD) after a myocardial infarction actually do so. The authors also found that older age did not appear to affect the mortality benefit that is associated with ICD implantation.
Multicentre study in patients shows idarucizumab reverses anticoagulant effect of dabigatran...
An interim analysis of the phase III RE-VERSE AD patient study demonstrates that 5g of the antidote idarucizumab reversed the anticoagulant effect of dabigatran within minutes in patients with serious bleeding complications or requiring urgent procedures.
Sorin and Cyberonics unveil name of combined company: LivaNova
Until the closing of the transaction, expected in the third calender quarter of 2015, both companies will continue to operate separately under their current brand names and leadership structures.
FDA approves trial to evaluate Lariat ligation of the left atrial...
The randomised, controlled AMAZE trial, will evaluate the use of the Lariat device for the ligation of the left atrial appendage as an adjunctive treatment to ablation in patients with persistent or long-standing persistent atrial fibrillation.
Medtronic announces US launch of Advisa SR MRI SureScan pacing system
The Advisa SR MRI SureScan single-chamber pacemaker with the 5076 MRI lead allows for magnetic resonance imaging (MRI) scans positioned on any region of the body without restrictions.
Andexanet alfa significantly reduces bleeding in a validated animal model using...
The reduction in blood loss correlated with reversal of the anticoagulant effects of rivaroxaban as measured by anti-Factor Xa activity.
First ESC recommendations for arrhythmias and chronic kidney disease published
The first ESC recommendations for patients with cardiac arrhythmias and chronic kidney disease (CKD) were presented at EHRA EUROPACE - CARDIOSTIM 2015 (21-24 June, Milan, Italy) and published in EP Europace.
Edoxaban approved in the European Union
Edoxaban (Lixiana, Daiichi Sankyo) is an oral, once-daily selective factor Xa-inhibitor for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors.
Fibrin and stem cell patches show promise for heart healing
findings of a study demonstrate the promise in regenerating cardiac tissue using engineered patches made up of a mixture of fibrin and mesenchymal stem cells (MSCs) derived from human umbilical cord blood.
Patients “test drive” pacemaker before choosing permanent implant
Patients are "test driving" a pacemaker outside the skin before deciding whether to have a permanent implant, according to research presented at EHRA EUROPACE-CARDIOSTIM by Michael Giudici, University of Iowa Hospitals and Clinics, USA.
ESC recommends uninterrupted vitamin K antagonists during ablation and device implantation
The recommendations are an update of EHRA's 2008 consensus document, which required an update due to dramatic changes in the field during the last five years.
Cecilia Linde
Cecilia Linde speaks to Cardiac Rhythm News about her work on a research platform for new onset heart failure in Stockholm, the highlights of this year's EHRA EUROPACE - Cardiostim Congress and her views on what is needed to improve cardiac care in Europe.
Boston Scientific initiates study to assess Emblem S-ICD in primary prevention...
The UNTOUCHED study will compare outcomes during an 18-month follow-up period to objective performance criteria derived from the MADIT-RIT study, which evaluated shock rates in 1,500 patients implanted with transvenous ICD devices.
Medtronic Micra transcatheter pacemaker meets initial safety and performance measures
The data from a global clinical trial involving the miniaturised device were presented at a late-breaking clinical trials session at EHRA EUROPACE-CARDIOSTIM 2015 (21-24 June, Milan, Italy).
One-year outcomes on stroke prevention in patients with newly-diagnosed atrial fibrillation...
Real-life data on stroke prevention from 17,200 patients will provide information on how patient risk profiles and quality of vitamin K antagonist control are associated with increased mortality and stroke in patients with newly diagnosed atrial fibrillation.
AliveCor announces availability of its Mobile ECG in Canada
The AliveCor Mobile ECG and the AliveECG app allows users to detect the presence of atrial fibrillation in an electrocardiogram (ECG or EKG) and manage their heart health with an electrocardiogram ECG monitor.
Medtronic acquires CardioInsight Technologies
CardioInsight Technologies will now become part of the Medtronic Atrial Fibrillation Solutions business in the Cardiac Rhythm and Heart Failure division.
Biosense Webster launches Carto 3 system Confidense module
The Confidense module's proprietary algorithm streamlines data collection, annotation and validation for the market-leading Carto system.
Benefit of anticoagulation in AF patients with a low risk of...
The results from a retrospective study, based on a large dataset (over 140,000 patients), have cast doubt on the benefit of routine oral anticoagulants for patients with atrial fibrillation and low risk of stroke.
Don’t miss Europe’s largest congress dedicated to cardiac arrhythmias
In June 2015, cardiac rhythm specialists will gather in Milan, Italy, to learn, share and discuss the latest advances in cardiac arrhythmias treatment in the largest European congress in the field: EHRA EUROPACE-CARDIOSTIM.
InvisionHeart receives FDA 510(k) clearance of its InvisionECG technology
The InvisionHeart ECG system provides a mobile solution for capturing and managing 12-lead ECGs, including the ability to read and visually compare, confirm, report and store diagnostic quality electrocardiograms.
AliveCor launches updated app providing Heart Journal
The Heart Journal is a feature that allows users to log and tag daily activities, symptoms and events in real-time that can impact heart health and work to identify abnormalities.
American Heart Association survery indicates potentially dangerous misconceptions about heart failure
The data from the American Heart Association will inform ongoing efforts and outreach about heart failure in the USA.
Baku 2015 equips European Games with Zoll AED Plus to treat...
Eighty AED Plus units will be distributed among the mobile medical teams and be available across all Baku 2015 venues.
Study published in The Lancet shows effective reversal of dabigatran in...
A study in healthy volunteers investigating the reversal of the anticoagulant effect of dabigatran etexilate (Pradaxa, Boehringer Ingelheim) by its specific agent idarucizumab has shown the antidote led to immediate, complete and sustained reversal of the anticoagulant effect.
Intravenous vernakalant facilitates electrical cardioversion in patients with cardioversion-resistant atrial fibrillation
intravenous vernakalant facilitated successful electrical cardioversion in patients who had failed to attain sinus rhythm following failed electrical cardioversion, or who immediately returned to atrial fibrillation after briefly attaining sinus rhythm.
Michael Gold
William Gold spoke to Cardiac Rhythm News about his involvement in various clinical trials, his views on the upcoming treatment options for heart failure and the highlights of this year's HRS meeting.
Report highlights urgent action needed to lessen the future impact of...
Report recommendations aim to improve management of atrial fibrillation as numbers are expected to double from 8.8 to 17.9 million adults aged over 55 years between 2010 and 2060.
Medtronic Tyrx antibacterial envelope reduces cardiac device infection rates at 12...
Long-term Citadel/Centurion clinical trial findings and independent data presented at Heart Rhythm Society 36th Annual Scientific Sessions.
Medtronic receives FDA approval and CE mark for Arctic Front Advance...
The third-generation cryoballoon is designed to allow enhanced positioning and help improve capture of real-time data with achieve mapping catheter.
New data show safety and efficacy of specifically designed implantable cardioverter...
Early performance results of the first-in-human international study of Medtronic's Micra Transcatheter Pacing System (TPS) have demonstrated the device is safe and effective.
First patient enrolled in dabigatran study comparing anticoagulation strategies during atrial...
The study assesses the safety and efficacy of uninterrupted anticoagulation with dabigatran etexilate (Pradaxa) during ablation procedures compared to warfarin. Results from the study are expected during 2016.
Cryoballoon ablation is not as safe as radiofrequency but is as...
Armin Luik presented findings at HRS 2015 indicating that cryoballoon ablation is a faster as effective as radiofrequency ablation for pulmonary vein isolation in paroxysmal atrial fibrillation patients, but has a greater complication rate and requires higher X-ray dosages.
Safety and efficacy of Transcatheter Pacing System demonstrated in initial study
Early performance results of the first-in-human international study of Medtronic's Micra Transcatheter Pacing System (TPS) have demonstrated the device is safe and effective.
Botox reduces AF burden for up to a year after cardiac...
Botulinum toxin injected into epicardial fat pads during coronary artery bypass graft surgery reduced the incidence of postoperative atrial fibrillation compared with placebo, with substantial suppression persisting after one year, a pilot study has found.
Cardiac resynchronisation is possible by leadless endocardial pacing of the left...
Results from the prospective, non-randomised, multicentre SELECT-LV trial were presented at the Heart Rhythm Society 36th Annual scientific Sessions and demonstrated promising efficacy and safety data.
Baroreflex activation therapy shows greater benefits in no-CRT heart failure patients
A phase II study onbaroreflex activation therapy treatment for heart failure patients with a reduced ejection fraction has found greater benefits for patients without a cardiac resynchronisation therapy device than for patients with one.
Activity after ICD implantation may predict survival
In the largest study on the relationship between activity and survival in ICD patients, researchers analysed how active participants were in the first 30-60 days after implantation and then over time up to four years.
Global trial finds Medtronic Micra transcatheter pacemaker meets initial safety measures...
All of the first 140 patients in the trial were successfully implanted with the Micra TPS. The data were presented at Heart Rhythm 2015 (13-16 May, Boston, USA).
Data shows advantages of Acutus Medical Dipole Density Heart Imaging and...
The data was presented at the Heart Rhythm Society's 36th Annual Scientific Sessions (13-16 May, Boston, USA) and shows the systems benefits compared with standard voltage-based mapping in patients with atrial flutter.
HeartLight US pivotal study meets primary efficacy and safety endpoints
The trial, which randomised CardioFocus' HeartLight one-to-one versus the Biosense Webster Thermocool catheter, met both primary efficacy and safety endpoints and demonstrated a low learning curve for physicians.
Abbott and GE Healthcare announce agreement to improve atrial fibrillation treatment
The agreement will bring real-time, patient-specific heart electrical activity data to cardiac electrophysiology labs around the world to speed up the diagnosis of the sources of atrial fibrillation and other heart rhythm disorders.
St Jude Medical expands portfolio of MRI-compatible devices to high voltage...
St Jude Medical has received CE mark approval of expanded labelling for its Ellipse implantable cardioverter defibrillator and its Durata and Optisure defibrillation leads. It has received CE mark approval for its Assurity MRI and Endurity MRI pacemaker device families.
Medtronic initiates study of in-office insertion of Reveal LINQ cardiac monitor
RIO 2 study evaluates safety and effectiveness of moving the insertion procedure from hospital to office setting.
First-in-human study backs neuromodulation to treat paroxysmal atrial fibrillation
A first-in-human study shows low level transcutaneous electrical vagus nerve stimulation, a completely non-invasive approach, suppresses paroxysmal atrial fibrillation.
No additional shocks or hospitalisations: Study shows home exercise safe after...
The results of a prospective, randomised trial demonstrate that moderately strenuous aerobic exercise, performed at home, for a select group of implantable cardioverter defibrillator (ICD) recipients was highly beneficial at improving cardiovascular performance. Importantly, the exercise did not compromise safety.
Biotronik gets CE mark approval for CardioMessenger Smart device to improve...
New device allows for earlier therapy adjustments in heart patients with pacemakers, implantable cardioverter-defibrillators, cardiac resynchronisation therapy and BioMonitor devices.
Bystander CPR helps cardiac arrest survivors return to work
In the largest study to date to examine return to work after cardiac arrest, researchers studied 4,354 patients in Denmark who were employed before they suffered out-of-hospital cardiac arrests between 2001 and 2011.
Japanese market release of Iperia 7 VR-T DX ProMRI
Japan is the world's largest MRI market with more MRI scanners per capita than any other country: approximately 47 registered machines per one million people.
New test predicts sudden cardiac death in haemodialysis patients
Patients with two or three of the predictors had a sudden cardiac death risk that was 145-times greater than patients with normal levels on all three measures.
Harvard Clinical Research Institute named as a data analytic centre for...
The PINNACLE Registry is a cardiological outpatient quality improvement registry, which collects data about patients in participating cardiology practices to help providers evaluate and improve their adherence to current guidelines.
Middle-aged congenital heart disease survivors may need special care
For the first time, the American Heart Association has issued recommendations for healthcare providers treating people older than 40 with congenital heart disease.
Atrial fibrillation recurrence lower with sleep apnoea treatment
The use of continuous positive airway pressure was associated with a significant reduction in the recurrence of atrial fibrillation in patients with obstructive sleep apnoea, according to a new analysis of data.
Long-term data show safety and efficacy of Boston Scientific’s S-ICD System
The S-ICD System was shown to convert more than 98% of heart arrhythmias that can lead to sudden death. These data are comparable to efficacy outcomes found in transvenous ICD (TV-ICD) clinical trials (95-99%).
FDA approves implantable cardioverter defibrillator with ultra-high energy on the first...
New Biotronik DX implantable cardioverter defibrillators with longer battery life and 42 joules on first shock offers "maximum energy", while the Itrevia implantable cardioverter defibrillator series is also approved.
Portola Pharmaceuticals announces positive phase 3 results from the ANNEXA-A study
Data support registration of Andexanet Alfa Bolus-Only and Bolus-Plus-Continuous-Infusion dosing regimens to reverse anticoagulant effect of Factor Xa Inhibitors.
CVRx Barostim Therapy clinical trial results to be presented at Heart...
Safety and efficacy results comparing patients previously treated with cardiac resynchronisation therapy to patients without cardiac resynchronisation therapy will be presented by Michael Zile from the Medical University of South Carolina, USA.
HeartWare achieves primary endpoint in the ENDURANCE destination therapy clinical trial
Investigators report data from first cohort in destination therapy at the 35th annual International Society for Heart and Lung Transplantation Meeting in Nice, France.
Edoxaban approved for prevention of stroke and systemic embolism in non-valvular...
Daiichi Sankyo has announced that Swissmedic, the regulatory authority of Switzerland, has granted approval of Lixiana (edoxaban), an oral, once-daily selective factor Xa inhibitor, for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation and treatment of venous thromboembolism in deep vein thrombosis.
Study re-examines sports restrictions for children with heart rhythm disorder
The study, published in JACC: Clinical Electrophysiology, found that the benefits of participation may outweigh risks for children with heart conditions.
Medtronic’s Micra Transcatheter Pacing System gets CE mark
The device is less than one-tenth the size of traditional pacemakers and is delivered with minimally invasive techniques through a catheter, and implanted directly into the heart.
AATS Graham Foundation and AtriCure announce James L Cox Fellowship in...
Fellowship provides newly-graduated cardiothoracic surgeons with a unique opportunity to be trained by nationally recognised experts in atrial fibrillation surgery.
Portola Pharmaceuticals announces positive results for phase 3 ANNEXA-A trial of...
Portola Pharmaceuticals has announced positive topline results from the second part of the phase 3 ANNEXA-A study, which evaluated the safety and efficacy of andexanet alfa, an investigational antidote, with the Factor Xa inhibitor apixaban.
Adding atrial fibrillation ablation to mitral valve surgery significantly increases risk...
A new study has found that a combination of surgical atrial fibrillation ablation and mitral valve surgery in patients with persistent or long-standing persistent atrial fibrillation is associated with a significantly increased rate of freedom from atrial fibrillation at one year. However, it is also associated with a significantly increased rate of pacemaker implantation.
Catheter ablation superior to amiodarone for patients with persistent atrial fibrillation...
Speaking at the 2015 American College of Cardiology Annual Scientific Session, Luigi Di Biase reported that-compared with the antiarrhythmic drug amiodarone-catheter ablation significantly increases the rate of freedom of recurrence in patients with persistent atrial fibrillation and heart failure.
First fully-implantable micropacemaker designed for foetal use
Researchers in the USA have developed the first fully-implantable micropacemaker designed for use in a foetus with complete heart block. The micropacemaker has been designated a humanitarian use device by the US Food and Drug Administration (FDA).
Biotronik announces first patient implanted with Eluna pacemaker system with full-body...
Biotronik has announced that the first US patient has been implanted with the company's Eluna pacemaker system with ProMRI technology.
Physically active middle-aged adults have low risk of sudden cardiac arrest
A review of 1,247 sudden cardiac arrest cases involving men and women ages 35-65 revealed that 63 cases (5%) were associated mainly with sports activities.
First patient enrolled in the GOLD AF registry of Medtronic’s PVAC...
Medtronic has announced the first patient enrolment in the GOLD AF registry, a first-of-its-kind, prospective, observational clinical study of its Phased Radiofrequency (RF) Ablation technology for treating patients with symptomatic atrial fibrillation.
Gender, age and mood disorders affect how patients perceive atrial fibrillation...
First-of-its-kind study published in HeartRhythm assesses various factors that lead to inaccurate detection of the frequency and duration of atrial fibrillation episodes.
Long-term sustained weight loss associated with significant reduction in AF burden
The first study investigating the long-term effects of weight loss and the degree of weight fluctuation on atrial fibrillation (AF) burden has found that obese patients with this arrhythmia who lost at least 10% of their body weight were six times more likely to achieve long-term freedom from atrial fibrillation.
FDA approves Biotronik Eluna pacemaker system with full-body ProMRI technology
Biotronik's ProMRI technology allows patients to undergo full-body magnetic resonance imaging (MRI) scans with both single-chamber and dual-chamber Eluna pacemakers when implanted with Setrox pacing leads.
CorMatrix CanGaroo ECM Envelope gets approval for South America
CorMatrix announced that it has received international approval from Argentina's National Administration of Drugs, Foods and Medical Devices (ANMAT) to market the CorMatrix CanGaroo ECM Envelope. canga
Arca biopharma provides GENETIC-AF clinical trial update
The GENETIC-AF phase 2B/3 clinical trial is evaluating Gencaro (bucindolol hydrochloride) as a potential treatment for atrial fibrillation.
Data shows ProMRI pacemakers to be safe and effective in full-body...
William M Bailey, medical director of Louisiana Heart Rhythm Specialists in Lafayette, discussed new clinical data at the American College of Cardiology's 64th Annual Scientific Sessions in San Diego.
Boston Scientific announces FDA and CE mark approval of the Emblem...
The Emblem S-ICD system is 19% thinner and is projected to last 40% longer than the previous system, improving patient comfort and cosmetic outcomes while reducing the number of times the device will require replacement.
Many atrial fibrillation patients at high risk of stroke fail to...
First phase II data demonstrating anticoagulant prescribing patterns in North America now available from GLORIA-AF Registry programme.
Cleveland Heart International and CU Medical roll out external defibrillator technology...
Cleveland Heart International to acquire majority interest in Korean automatic external defibrillators manufacturer CU Medical.
ResMed to present data on sleep-disordered breathing in chronic heart failure...
Data from the two studies will be presented at the 64th Annual Scientific Sessions of the American College of Cardiology (ACC) (14-16 March, San Diego, USA).
ACC releases updated training recommendations for cardiology fellows
This latest version defines cardiology core competencies for the first time.
Societies release revised training guidelines for paediatric cardiology fellowship programmes
The Society of Pediatric Cardiology Training Program Directors, the American College of Cardiology, the American Academy of Pediatrics and the American Heart Association worked to develop the guidelines.
First in-man study of miniaturised implantable cardiac monitor shows positive results
First in-man multicentre study of the miniaturised implantable cardiac monitor Reveal Linq (Medtronic) suggests the device is safe and provides adequate sensing capabilities to detect arrhythmias.
AliveCor launches new app that automatically tells patients when their ECG...
Anytime an ECG is taken with the AliveCor Heart Monitor, the AliveECG app will tell users if atrial fibrillation is present, if the ECG is normal, or if there is too much interference and another ECG should be taken.
Zoll ResQCPR system receives premarket approval from the FDA
The ResQCPR system demonstrated a 49% increase in one-year survival in adult patients who experienced out-of-hospital cardiac arrest of presumed cardiac etiology, as compared to treatment with conventional manual CPR.
TomTec receives FDA 510(k) clearance for its latest version of TomTec-Arena...
TomTec-Arena is a suite of clinical applications to review, analyse and quantify medical image data in multiple dimensions (2D and 3D/4D) and multiple modalities.
Updated US guidelines suggest an increase by 19% in AF patients...
Two-thirds of patients who were not previously recommended for oral anticoagulation therapy are now recommended, according to an assessment of the 2014 AHA, ACC and HRS atrial fibrillation guidelines.
Atrial fibrillation patients on digoxin face increased risk of death
Analysis of 19 studies finds atrial fibrillation patients taking digoxin are 27% more likely to die than those who do not.
Global atrial fibrillation treatment market to peak at US$9.4bn in 2020
The market will more than double in value from US$4.6bn in 2013 to US$9.4bn in 2020, before declining to US$5.7bn by 2023, according to research and consulting firm GlobalData.
London Bridge Hospital partners with Quail Digital to enhance clinical communications...
Quail's wireless headset system will provide hands-free communications between the treatment lab and monitoring room, which are separated by insulated partitions and glass.
Boehringer Ingelheim submits biologics license application to FDA for reversal agent...
Boehringer Ingelheim has announced it has submitted a biologics license application to the FDA, requesting an Accelerated Approval pathway, for the use of idarucizumab to reverse the anticoagulant effect of dabigatran.
Cyberonics and Sorin announce merger plan
Sorin and Cyberonics have announced their merger plan with a combined equity value of approximately US$2.7bn (€2.4bn).
Spinal cord stimulation in severe heart failure patients is safe and...
Results of a prospective, multicentre pilot trial of severe heart failure patients treated with continuous spinal cord stimulation have shown the treatment is safe and feasible with the Eon Mini neurostimulation device (St Jude Medical) and can potentially improve symptoms, functional capacity and cardiac function.
Vitaria vagal nerve stimulation system receives CE mark for treatment of...
Vitaria delivers autonomic regulation therapy for patients who have moderate to severe heart failure with left ventricular dysfunction (ejection fraction < 40%), and who remain symptomatic despite stable, optimal heart failure drug therapy.
Subcutaneous-ICD patients may be able to undergo MRI scans at 1.5T...
Subcutaneous implantable cardioverter-defibrillator patients may be safe to be exposed to magnetic resonance imaging (MRI) procedures at 1.5T with a pre-specified scanning and monitoring protocol.
Heart failure patients who struggle with daily tasks are hospitalised more...
The risk is higher for older women, unmarried people and those with chronic conditions that affect mobility and ability, including obesity, dementia, anaemia and diabetes, researchers said.
First patients enrolled in DEEP clinical study
The study is the first-of-its-kind in the USA exploring a minimally invasive epicardial surgical ablation approach combined with an endocardial catheter-based ablation approach for the treatment of persistent or long-standing persistent atrial fibrillation.
Heart-failure helpline could be “difference maker” for turning disease into a...
The Stronger Hearts Helpline pilot programme centralises resources to educate and improve access to care.
CHU de Saint- Étienne launches second stereotaxis lab
With the launch, the hospital becomes the first in Europe to operate two Niobe systems for clinical procedures.
New data shows iRhythm reduces cost of arrhythmia detection compared to...
The study, which was funded by iRhythm, suggests that the lack of diagnosis after the initial use of the Holter monitor resulted over US$23,000 per patient in wasted spending by Medicare.
ProMRI studies confirm safety of MR imaging with Biotronik devices
Studies validate safety of Biotronik ProMRI devices in patients subjected to head and lower lumbar 1.5T MRI scans.
Real-world study shows effectiveness of Reveal LINQ to detect atrial fibrillation...
New data demonstrate cost-effectiveness of long-term, continuous cardiac monitoring with potential to prevent recurrent strokes.
Stem cells from placenta show promise for treating heart failure
A new study has examined the therapeutic effects of PDA-001, an intravenous formulation of PDAC cells, in mice, as well as the best way to deliver the therapy.
Sorin announces enrolment of first patients in the Vanguard clinical study
The Vanguard (Vagal nerve stimulation safeguarding heart failure patients) clinical study evaluates Equilia, a neurostimulation system for heart failure patients.
Real-world multicentre study shows positive outcomes with left atrial appendage occlusion...
The largest study using the Amplatzer Cardiac Plug (St Jude Medical) for left atrial appendage occlusion has shown a high procedural success rate and favourable outcomes for prevention of atrial fibrillation-related thromboembolism.
Hansen Medical announces expanded protocol for the ARTISAN-AF IDE study
The amended protocol now includes the use of the Thermocool SmartTouch catheter (BioSense Webster) and the EnSite Velocity Cardiac Mapping System (St Jude Medical) in combination with the Sensei Robotic System (Hansen Medical) for the treatment of paroxysmal atrial fibrillation.
FDA approves Savaysa for reduction of stroke risk in non-valvular atrial...
Savaysa (edoxaban) is an oral, once-daily selective factor Xa inhibitor, designed to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation
Wearable sensor smoothes path to long-term ECG and EMG monitoring
The new sensor is as accurate as the "wet electrode" sensors used in hospitals, but can be used for long-term monitoring and is more accurate than existing sensors when a patient is moving.
Phase 3 ANNEXA-R study of andexanet alfa and factor Xa inhibitor...
Andexanet alfa is the only universal factor Xa inhibitor antidote shown to directly reverse the anticoagulant activity of these agents in clinical studies.
Hung-Fat Tse
While Hung-Fat Tse's initial research interests focused on device therapy for cardiac arrhythmias, he has dedicated his career to the development of cardiovascular regenerative medicine.
Boston Scientific launches long-lasting implantable cardioverter defibrillators
The devices feature EnduraLife battery technology developed with high-performance chemistry and advanced manufacturing capabilities to provide up to double the battery capacity of other defibrillators.
CVRx Barostim Therapy trial results to be presented at the ACC...
CVRx completed enrolment of the 146-patient clinical trial to determine the performance of Barostim Therapy for patients suffering from chronic heart failure with advanced symptoms.
FDA clears AliveCor’s new automated detectors for normal ECG recordings and...
AliveCor's new algorithms include a Normal Detector that identifies when no abnormalities are detected in an ECG recording and an Interference Detector that identifies if factors have affected the recording making the ECG unreadable.
Bridging during anticoagulation interruption associated with higher bleeding risk in AF...
A subanalysis from the ORBIT-AF registry has found that atrial fibrillation patients who receive bridging anticoagulation therapy during anticoagulation interruption are at higher risk of bleeding and adverse events following interruption than patients who do not receive bridging anticoagulation.
Bayer collaborates on phase III study to evaluate Xarelto in embolic...
In addition, Xarelto (rivaroxaban) label now includes guidance for use in patients with atrial fibrillation undergoing cardioversion.
St Jude Medical announces FDA approval of FlexAbility Ablation Catheter
The FlexAbility catheter features a handle and shaft combination that allows for improved maneuverability, enabling electrophysiologists to reach challenging anatomic locations within their patients' hearts.
AliveCor launches atrial fibrillation algorithm detector for its Heart Monitor
The latest version of the AliveECG app for users in the United Kingdom and Ireland now provides patients with real-time atrial fibrillation detection in electrocardiogram recordings using the AliveCor Heart Monitor.
Ten per cent of heart patients may be inappropriately prescribed aspirin
Accessing data from the National Cardiovascular Disease Registry PINNACLE Registry, researchers examined a nationwide sample of 68,808 patients receiving aspirin for primary cardiovascular disease prevention.
Biotronik concludes enrolment in phase C of its ProMRI ICD Study
The ProMRI study consists of a series of MRI compatibility trials intended to increase cardiac device patients' access to MRI.
Milestone Pharmaceuticals receives FDA clearance of MSP-2017 phase 2 IND
The trial design cleared by FDA is a multiple arm phase 2, multicentre, placebo-controlled study of intranasal administration of MSP-2017 for the conversion of PSVT to sinus rhythm.
St. Jude Medical begins study of ablation procedures to treat ventricular...
The FlexAbility ablation catheter will be used to assess benefits of catheter ablation as an adjunctive therapy for patients at risk for life-threatening arrhythmias.
Medtronic initiates European trial to evaluate cryoballoon ablation treatment for atrial...
Cryoablation creates lesions by freezing tissue in the heart's upper chambers, traditionally around the pulmonary veins, to block the electrical signals that trigger erratic heart rhythms.
Medtronic announces first patient enrolment in Tyrx absorbable antibacterial envelope trial
The trial will evaluate the effectiveness of the Tyrx absorbable antibacterial envelope in reducing major infections in patients with cardiac implantable electronic devices at risk of infection.
Stereotaxis receives FDA clearance of Vdrive system and Japanese regulatory approval...
The Vdrive allows physicians to remotely control the advancement, retraction and rotation of a compatible fixed curve transseptal sheath, in conjunction with a magnetic ablation catheter.
Abbott completes acquisition of Topera
Abbott has acquired all outstanding equity of Topera for US$250m upfront, plus potential future payments tied to performance milestones.
Biosense Webster launches programme to reinforce patient benefits of company’s contact...
The Biosense Webster Advantage programme is an outcomes-based, risk-sharing program for US hospitals that reinforces the significant patient benefits demonstrated by the ThermoCool SmartTouch Catheter.
AliveCor launches third generation heart monitor
AliveCor says that the third generation device is 50% thinner and 40% lighter and was designed from first-hand customer feedback and in collaboration with global design firm IDEO.
Philips’ defibrillators to be made available to schools across the UK
Philips secures nationwide Department for Education tender to provide schools with the latest HeartStart FRx automated external defibrillators.
REPLACE DARE score provides tool for risk stratifying pacemaker or implantable...
Analysis of Biotronik-sponsored REPLACE registry identifies key patient risk factors and reveals comorbidities more relevant than procedure-related complications.
The challenge of left ventricular lead placement: What is the role...
George H Crossley (Nashville, USA) writes on the role of quadripolar leads in cardiac resynchronisation therapy to treat heart failure patients.
Non-invasive mapping prior to ablation may be an effective strategy for...
Preliminary data from the AFACART study have shown encouraging results when treating persistent atrial fibrillation with non-invasive mapping followed by pulmonary vein isolation and/or linear lesions.
First trial of oral anticoagulant to prevent recurrent stroke due to...
The first patient has been enrolled in the RE-SPECT ESUS phase III study to investigate the efficacy and safety of dabigatran etexilate for the prevention of recurrent embolic stroke of undetermined source.
Guidelines added to ACC Guideline Clinical App
The American College of Cardiology's recently-released Guideline Clinical App has expanded with the addition of tools and guideline content for valvular heart disease and atrial fibrillation.
St Jude Medical receives CE mark approval of the Allure Quadra...
The Allure Quadra MP cardiac resynchronisation therapy pacemaker contains MultiPoint Pacing technology, which enables physicians to pace multiple locations on the left ventricle, giving the clinician more choices to best optimise CRT pacing based on patient need and reducing the rate of CRT non-responders.
Medtronic receives FDA approval and launches additional Attain Performa leads
The newest additions to the Attain Performa lead portfolio are designed to accommodate patients' varying vessel sizes and curvatures to enhance successful lead placement.
Biotronik announces conclusion of full-body ProMRI pacemaker study
Phase B of Biotronik's ProMRI study has completed patient enrolment and concluded all planned scans.
New research reports low rate of mechanical failure in Optim family...
A new study evaluating Optim-insulated implantable cardioverter defibrillator leads found low rates of all-cause mechanical failure during a median follow-up of 3.2 years.
iRhythm Technologies receives CE mark for ZIO Service and enters UK...
iRhythm Technologies has secured a CE mark for the ZIO Service, enabling the company to market its long-term continuous heart monitoring solution in Europe.
Biotronik launches new ProMRI ICD and CRT-D series with Sentus QP...
Biotronik has launched its new series of single- and dual-chamber implantable cardioverter defibrillators and cardiac resynchronisation therapy defibrillators.
Epinephrine may do more harm than good for cardiac arrest patients
For patients in cardiac arrest, administering epinephrine may increase the overall likelihood of death or debilitating brain damage, according to a study published in the Journal of the American College of Cardiology.
Ventricular tachycardia treatment with robotic catheter ablation shows positive results
A study undertaken in the UK has shown that radiofrequency ablation of scar-related ventricular tachycardia, using robotic navigation, is safe and feasible and has resulted in 95% reduction in total implantable cardioverter defibrillator (ICD) therapy burden at six months.
European cardiologists highlight risk of delayed diagnosis for patients with non-valvular...
A new survey has found that despite an increase in the number of non-valvular atrial fibrillation patients compared to five years ago, the majority of cardiologists believe there is a delay in patients reaching diagnosis.
Investigational idarucizumab may reverse effect of dabigatran, new data show
New data on the investigational antidote idarucizumab show that it may reverse the effect of the oral anticoagulant dabigatran (Pradaxa, Boehringer Ingelheim) on both blood coagulation and the blood clotting mechanism.
Drug helps lower high potassium levels associated with potentially lethal cardiac...
Mikhail Kosiborod, of Saint Luke's Mid America Heart Institute, Kansas City, and colleagues evaluated the efficacy and safety of the drug zirconium cyclosilicate in patients with hyperkalemia (higher than normal potassium levels).
Biotronik releases new online tool to track MR conditional status of...
Biotronik has launched ProMRI SystemCheck, a new online tool for tracking the ProMRI status of implantable devices.
Long-term overtreatment with antiplatelet/anticoagulant therapy may increase the risk of dementia...
Atrial fibrillation patients who are receiving a combination of antiplatelet and anticoagulant therapy and are over treated with warfarin may be at an increased risk of dementia, according to a study presented at the American Heart Association's Scientific Sessions (AHA;15-19 November, Chicago, USA).
Algisyl-LVR shows significant health status improvement of advanced heart failure patients
LoneStar Heart has presented the primary six month results of its multicentre, randomised clinical trial of Algisyl-LVR, providing evidence that the cardiac hydrogel implant can prevent or reverse the symptoms of moderate to severe heart failure in patients with a dilated and weakened left ventricle.
CardioMEMS HF significantly reduces 30-day hospital readmission rates
Retrospective data analysis from the CHAMPION clinical trial shows significant reduction in 30-day hospital readmission rates for heart failure patients age 65 and older treated with the CardioMEMS HF system (St Jude Medical).
Studies show Medtronic CRT devices reduce readmissions after heart failure hospitalisations
New data from Medtronic show that its cardiac resynchronisation therapy devices for the treatment of heart failure can cause a significant reduction in all-cause 30-day readmissions after heart failure hospitalisations.
Highlights from ISCAT 2014
Michel Haissaguerre and Etienne M Aliot, chairmen of the International Symposium on Catheter Ablation Techniques (ISCAT; 15-17 October 2014, Paris, France), discuss the highlights of this year's meeting with Cardiac Rhythm News.
New positive data from investigational drug (LCZ696) for heart failure presented...
New data from PARADIGM-HF have shown that the investigational angiotensin receptor-neprilysin inhibitor (ARNI) LCZ696 (Novartis) has the potential to prevent the clinical progression of surviving patients with heart failure more effectively than enalapril.
Biosense Webster and StopAfib.org work to raise awareness of AFIB
Biosense Webster has partnered with advocacy organisation StopAfib.org to encourage Americans to "Get SMART About Afib."
Watchman presents possible alternative to warfarin for stroke prevention in patients...
Study examines the long-term efficacy and safety of Boston Scientific's Watchman device to achieve left atrial appendage closure in patients with atrial fibrillation.
Study recommends repeat testing of Brugada syndrome patients
A study has investigated the clinical significance of repeat testing after puberty in asymptomatic children with a family history of Brugada syndrome who had an initial negative test earlier in childhood.
Survey uncovers need to raise awareness of link between atrial fibrillation...
A survey issued by the Heart Rhythm Society and National Stroke Association in collaboration with Boehringer Ingelheim has found there are information gaps regarding the impact of AF-related stroke - among AF patients, physicians and caregivers - including communication barriers, challenges with patient education, misperceptions about treatment compliance, and outcomes related to the impact of stroke on one's life.
Stressful duties linked with risk of sudden cardiac death among police...
Stressful and physically-demanding law enforcement activities are associated with large increases in the risk of sudden cardiac death among US police officers compared with routine policing activities.
S-ICD system receives favourable US coding and payment designations for 2015
Boston Scientific has announced that its subcutaneous implantable defibrillator (S-ICD System) will have designated Current Procedural Terminology (CPT) Category I codes by the American Medical Association, effective 1 January 2015.
MiD study unveils key arrhythmia findings in patients with end-stage renal...
Medtronic has announced the results of a new study which found that atrial fibrillation and bradycardia occurred at higher than expected, and clinically significant, rates in patients with end-stage renal disease undergoing haemodialysis.
American College of Cardiology announces launch of JACC: Clinical Electrophysiology
The American College of Cardiology has announced the launch of the Journal of the American College of Cardiology (JACC): Clinical Electrophysiology, which will feature original research and review articles regarding cardiac rhythm disorders.
The challenge of catheter ablation in congenital heart disease patients
Dominic Abrams (Division of Cardiac Electrophysiology, Boston Children's Hospital, Boston, USA) writes on the importance of arrhythmia management in congenital heart disease patients and identifies the reasons why it remains a major challenge for interventional eletrophysiology.
Continuous ECG surveillance of marathon athletes is feasible
Online electrocardiogram (ECG) continuous surveillance during marathon running is feasible, according to a study presented at the European Congress on e-Cardiology and e-Health (29-31 October, Bern, Switzerland). The proof-of-concept study may allow "instantaneous diagnosis of potentially fatal rhythm disorders" in endurance athletes.
Antiarrhythmic drug vernakalant shows positive results in Asia-Pacific phase 3 clinical...
The study achieved the primary endpoint, showing that 53% of patients with recent onset atrial fibrillation (lasting three hours to seven days) receiving an intravenous dose of vernakalant converted to normal heart rhythm within 90 minutes, compared to 12% of placebo patients.
AtriCure receives FDA approval to start DEEP IDE study
The DEEP study will investigate a minimally invasive approach performed by cardiac surgeons and electrophysiologists to treat patients with persistent or long-standing persistent atrial fibrillation who have failed antiarrhythmic drug therapy.
ACC launches new Guideline Clinical App for cardiologists, general practitioners
The American College of Cardiology (ACA), which has been developing clinical practice guidelines in partnership with the American Heart Association (AHA) for more than three decades, has launched a new Guideline Clinical App to serve as the mobile home for all ACC/AHA guideline content and related tools.
LifeWatch signs agreement to utilise Vital Connect’s HealthPatch MD
LifeWatch AG has signed an agreement with Vital Connect Inc. to utilise its HealthPatch MD as a 1-lead ECG device in its cardiac monitoring business.
Dabigatran associated with higher incidence of major bleeding than warfarin
A study of Medicare beneficiaries suggests that dabigatran (Pradaxa, Boehringer Ingelheim) should be prescribed with caution, especially among high risk patients, as it may be associated with a higher incidence of major bleeding and a higher risk of gastrointestinal bleeding, despite a lower risk of intracranial haemorrhage than warfarin, according to a study published online in JAMA Internal Medicine.
FDA makes recommendation on Daiichi Sankyo’s Savaysa
Daiichi Sankyo has announced that the FDA has recommended approval of once-daily Savaysa (edoxaban) 60mg dosing regimen.
Hansen Medical announces global launch of the Sensei X2 robotic system
Hansen Medical's Sensei X2 robotic system is now commercially available in the USA and European Union.
St Jude Medical receives FDA approval of TactiCath Quartz Contact Force...
St Jude Medical has announced the US Food and Drug Administration (FDA) approval of its TactiCath Quartz irrigated ablation catheter, the company's newest technology that gives physicians a real-time, objective measure of the force that the catheter applies to a patient's heart wall during an ablation procedure.
Vitamin D deficiency increases poor brain function after cardiac arrest
Vitamin D deficiency increases the risk of poor brain function after sudden cardiac arrest by seven-fold, according to research presented at Acute Cardiovascular Care 2014 by Jin Wi.
Heart rate may predict survival and brain function in comatose cardiac...
A study has found that patients with sinus bradycardia during therapeutic hypothermia had a 50-60% lower mortality rate at 180 days than those with no sinus bradycardia.
Abbott to acquire Topera and Advanced Cardiac Therapeutics
Abbott enters the catheter-based electrophysiology market with the announcement of an agreement to purchase Topera and the securement of the rights to purchase Advanced Cardiac Therapeutics (ACT). Both companies develop electrophysiology technologies for the diagnosis and treatment of heart rhythm disorders.
Persistent atrial fibrillation treatment with robotic navigation and contact force sensing...
An international multicentre registry evaluating the role of remote robotic navigation and contact force sensing in persistent atrial fibrillation (AF) treatment has found that, at one year, a higher proportion of patients treated with this modality were free from AF recurrences with a single procedure as compared with both manual/contact force sensing and robotic/no contact force sensing procedures.
Living near major roads may increase risk of sudden cardiac death...
A new study has found that living close to a major road may increase women's risk of dying from sudden cardiac death.
Boston Scientific receives CE mark approval for MRI compatible Accolade pacemakers...
Boston Scientific has announced the CE mark approval for the Accolade pacemaker family and for the Visionist and Valitude cardiac resynchronisation therapy pacemakers (CRT-P) with quadripolar pacing technology.
Medtronic gets FDA approval of pacing lead for full-body MRI scans
Medtronic has announced the US Food and Drug Administration (FDA) approval of its CapSureFix Novus MRI SureScan 5076 lead for use with magnetic resonance imaging (MRI).
Massachussets General Hospital to coordinate new global research initiative in USA...
The Massachusetts General Hospital Institute for Heart, Vascular and Stroke Care has announced participation as coordinating centre in the USA for a new research initiative dedicated to explore the genetic causes of atrial fibrillation as part of the Transatlantic Network of Excellence in Cardiovascular and Neurovascular Research Program.
AtriCure completes enrolment in post approval study for the Synergy Ablation...
AtriCure has announced that enrolment in the ABLATE post approval study (PAS) of its Synergy Ablation System is complete. As 3 October 2014, the ABLATE PAS enrolled 365 patients at 40 hospitals across the United States.
Massimo Santini
Massimo Santini performed the first fulguration of the atrioventricular node of resistant supraventricular tachycardia in Italy; he also implanted a pacemaker in the Italian president Carlo Azeglio Ciampi, 10 years ago. Santini speaks to Cardiac Rhythm News about his views on advances in antiarrhythmic drugs, genetics and cardiac arrhythmias, and ECG screening.
FDA’s Advisory Committee Panel votes in favour of Watchman device
Members of the US Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee have voted in favour of the Watchman left atrial appendage closure (LAAC) device (Boston Scientific), by a close vote of 6 to 5 (with 1 abstention) concluding that the potential benefits of Watchman outweigh the potential risks. The Panel also voted unanimously in favour of the device safety; however, its effectiveness saw unfavourable voting.
Novel protein in heart linked to ventricular arrhythmias
Cardiovascular scientists in the USA have identified in mouse models a protein known as Purkinje cell protein-4 (Pcp4) as a regulator of the heart's rhythm. Additionally, they have found that when the Pcp4 gene is disrupted, it can cause ventricular arrhythmias.
CVRx announces first successful Barostim neo heart failure commercial implants in...
CVRx has announced the first 10 heart failure patients treated with the Barostim neo system under CE mark approval.
Radiation risks should be considered and discussed before heart imaging: American...
Before undergoing heart imaging procedures involving radiation, healthcare providers should help patients understand why the procedure is needed and its potential benefits and risks, including risks related to radiation exposure, according to a new scientific statement in the American Heart Association's journal Circulation.
Societies release first appropriate use criteria for paediatric heart disease
The American College of Cardiology, along with eight partnering societies, have released the first appropriate use criteria for suspected heart disease in paediatric patients.
Try something new this world heart day, urges the ESC
Reflecting on the World Heart Day, spokespersons from ESC call for the need to encourage cardiovascular disease prevention through healthy diets and increment of physical activity.
AtriCure launches AtriClip Flex for left atrial appendage occlusion
Atricure has announced the introduction of the AtriClip Flex, a new device used in left atrial appendage occlusion for stroke prevention in atrial fibrillation patients. According to a company release, the device has a more flexible aluminum shaft that allows surgeons to better manoeuvre within a patient's particular anatomy.
St Jude Medical receives CE mark approval for pacing lead labelling...
St Jude Medical has announced CE mark approval of updated labelling for its Tendril STS and IsoFlex Optim pacing leads. The new labelling allows existing and future patients with the devices access to magnetic resonance imaging (MRI) scans.
First randomised sham controlled trial of vagal nerve stimulation for heart...
NECTAR-HF, the first randomised, sham-controlled trial of vagal nerve stimulation for the management of heart failure has failed to show significant difference in cardiac remodelling between vagal nerve stimulation and a sham procedure-meaning that the study has not met its pre-specified six-month primary efficacy endpoint.
AHA and ACC release scientific statement on congenital and genetic heart...
The American Heart Association (AHA) and American College of Cardiology (ACA) have released a statement including 14 key elements that can be used as a checklist for screening young people age 12-25 for congenital and genetic heart disease.
New data analysis from CHAMPION shows significant reductions in heart failure...
St Jude Medical has announced a new data analysis from the CHAMPION clinical trial-in a subgroup of patients with renal failure-that showed reduced heart failure hospitalisation by 42% for patients managed with pulmonary artery pressure based technology compared to a control group.
Medtronic launches Seeq wearable cardiac monitoring system in USA
Medtronic has announced the US launch of the Seeq Mobile Cardiac Telemetry (MCT) System, an external, wire-free, adhesive heart monitor that can be worn for up to 30 days to help detect and diagnose the cause of irregular heartbeats.
Rivaroxaban may be a safe alternative to standard of care in...
Results from the X-VERT trial have shown that rivaroxaban is a safe and effective alternative to Vitamin K antagonist (VKA) therapy in patients with atrial fibrillation who are undergoing elective cardioversion.
Preventice and eCardio merge as a new holding company
Preventice, developer of the PatientCare Platform and the BodyGuardian Remote Monitoring Sensor and eCardio, a provider of remote cardiac monitoring products and services, have announced a strategic combination to drive innovation and growth in remote monitoring systems and mobile health applications.
Permanent AF doubles risk of stroke compared to paroxysmal AF
Permanent atrial fibrillation (AF) doubles the risk of stroke compared to paroxysmal AF, according to research in more than 6,000 patients presented at European Society of Cardiology Congress. The findings suggest that a simple clinical assessment of the type of AF can help doctors to better estimate stroke risk.
Sudden death predictor identifies ICD candidates in new ESC Guidelines
A new sudden death predictor for patients with hypertrophic cardiomyopathy identifies candidates for implantable cardioverter defibrillators (ICDs) in ESC Guidelines published recently. They were presented at the European Society of Cardiology (ESC) Congress by Task Force chairperson Perry Elliott (The Heart Hospital, London, UK).
Remote monitoring costs in ICD patients comparable to in-office monitoring
Results from the EuroEco trial have found that the cost of remote monitoring (with Biotronik Home Monitoring) of patients with implantable cardioverter defibrillators (ICDs)-to physicians, hospitals and insurance providers-does not differ significantly from traditional in-office monitoring.
Favourable effect of Pradaxa on kidney function over time compared to...
New data indicate that kidney function decline is less pronounced in patients with non-valvular atrial fibrillation who are treated with Pradaxa (dabigatran etexilate) compared to warfarin.
FDA clears Vdrive with V-Loop system
Stereotaxis has announced that it has received 510(k) clearance by the Food and Drug Administration (FDA) to market its Vdrive robotic navigation system with V-Loop variable loop catheter manipulator in the USA.
New drug LCZ696 superior to enalapril in heart failure treatment
Findings from the PARADIGM-HF trial have found that the investigational medicine LCZ696 (Novartis) cuts cardiovascular deaths by 20% compared to ACE inhibitor enalapril in heart failure patients with reduced ejection fraction.
Experience in clinical practice and expanding research programme support use of...
During this final phase of the registry programme, data on the overall safety and effectiveness of antithrombotic treatments will be collected.
IN-TIME trial confirms 50% reduction in mortality in HF patients
The IN-TIME trial, which was recently published in The Lancet, demonstrated that heart failure patients' mortality can be reduced by more than 50% using Biotronik Home Monitoring.
Large-scale study confirms growing body of clinical evidence for St Jude...
St Jude Medical has announced results from a large-scale, clinical study concluding that the Quartet left-ventricular quadripolar lead provides more options to effectively manage common pacing complications compared to systems with bipolar leads.
Treating cardiac patients who have sleep apnoea with positive airway pressure...
Results from an analysis of the German Statutory Insurance database showed that the three-year mortality of people with sleep apnoea was significantly lower in patients who were treated with positive airway pressure devices compared to a similar cohort who were not.
BioControl Medical crosses enrolment milestone in INOVATE-HF study
BioControl Medical has announced that it has reached 480 randomised patients - or 70% - of the planned 650 subjects with congestive heart failure in the INOVATE-HF trial of its CardioFit system.
SMART-AF trial shows AF ablation with contact force sensing catheter is...
Results from the SMART-AF trial, recently published in the Journal of the American College of Cardiology, have shown that the ThermoCool SmartTouch contact force-sensing catheter is safe and effective for the treatment of drug-refractory symptomatic paroxysmal atrial fibrillation.
First implants in largest-ever global trial of cardiac resynchronisation therapy
Medtronic has announced the first implants in a clinical trial that will compare patient and healthcare system outcomes in heart failure patients who have cardiac resynchronisation therapy (CRT) devices with the AdaptivCRT feature enabled versus patients receiving standard CRT.
Joint consensus on antithrombotic use in AF patients with acute coronary...
A new European joint consensus document on the use of antithrombotic drugs in patients with atrial fibrillation presenting with an acute coronary syndrome and undergoing percutaneous coronary intervention has been published in the European Heart Journal.
FDA clears CorMatrix CanGaroo ECM envelope
CorMatrix Cardiovascular has announced that it has FDA clearance to market the CorMatrix CanGaroo ECM envelope for use with cardiac implantable electronic devices, including pacemakers and implantable cardioverter defibrillators (ICD's).
Medtronic Viva CRT-P gets FDA approval
Medtronic has announced the US Food and Drug Administration (FDA) approval of its newest cardiac resynchronisation therapy-pacemaker, Viva CRT-P, for indicated patients with heart failure or atrioventricular block.
Study finds nMARQ ablation catheter fast and effective for the treatment...
A recent feasibility study has found irrigated multi-electrode radiofrequency ablation of atrial fibrillation using the novel 10-pole circular, open-irrigated mapping and ablation catheter nMARQ device (Biosense Webster) to be fast and effective.
AliveCor receives first FDA clearance to detect a serious heart condition...
AliveCor has announced that the US Food and Drug Administration (FDA) has granted the company clearance for its algorithm to detect atrial fibrillation.
Bristol-Myers Squibb and Pfizer to present new Eliquis (apixaban) data at...
Bristol-Myers Squibb Company and Pfizer have announced that they will present 14 abstracts (oral and poster presentations) at the ESC Congress 2014, 30 August to 4 September in Barcelona, Spain.
John Muir Health’s Concord medical centre one of 20 hospitals in...
John Muir Health's Concord medical centre is one of just 20 hospitals in the USA to begin treating patients who suffer from atrial fibrillation with a new procedure that features the FDA-cleared FIRMap catheter.
Impulse Dynamics announces first implantation of the Optimizer IVs device in...
Impulse Dynamics has announced the first implantation of the Optimizer IVs device in the Czech Republic earlier this summer. The implantation took place at the Na Homolce Hospital in Prague (Czech Republic) by Petr Neuzil, head of Cardiology at the same institution.
Exercise may protect older women from atrial fibrillation
Increasing the amount or intensity of physical activity can cut the chances of older women developing atrial fibrillation, according to new research in the Journal of the American Heart Association (JAHA).
Hansen Medical announces exclusive distribution with Adachi
Hansen Medical and Adachi have announced a distribution agreement for Magellan and Sensei Robotic Systems in Japan. As part of the distribution agreement, Adachi is responsible for obtaining Japanese Marketing approval from the Pharmaceuticals and Medical Devices Agency for the systems.
PARADIGM-HF trial results of LCZ696 for heart failure to be presented...
New data revealing the reduction in cardiovascular deaths with Novartis' LCZ696 in patients with heart failure with reduced ejection fraction will be presented at the European Society of Cardiology (ESC) Congress 2014.
IN-TIME trial results on remote monitoring published in The Lancet
Biotronik has announced that the IN-TIME study results have been published in The Lancet. The randomised, controlled trial is the first worldwide to demonstrate that automatic, implant-based remote monitoring leads to significant clinical benefits for heart failure patients.
Lariat device shows high rate of acute left atrial appendage closure...
A first US multicentre registry with the Lariat device (SentreHeart) for left atrial appendage ligation has shown 94% acute closure rate; however, procedural success has been limited with major bleeding.
Sports for young patients with implantable cardioverter defibrillators: Refining the risk
Elizabeth Saarel (University of Utah, Salt Lake City, USA) argues that, based on prospective registry data, young patients with implantable cardioverter defibrillators can participate safely in competitive sports.
FDA approves Attain Performa quadripolar lead and Viva Quad CRT-Ds
Medtronic has received US Food and Drug Administration (FDA) approval for the Attain Performa Model 4298 quadripolar lead, and the Viva Quad XT and Viva Quad S cardiac resynchronisation therapy defibrillators (CRT-D).
CardioMEMS system receives approval from CMS for New Technology Add-On Payment
St Jude Medical has announced that the Center for Medicare and Medicaid Services (CMS) has approved a New Technology Add-on Payment (NTAP) for the CardioMEMS heart failure system.
Societies mark 30 years of guidelines for evidence-based cardiovascular care
Marking the 30th anniversary of the publication of their first joint guidelines for the diagnosis and treatment of heart disease, the American College of Cardiology and the American Heart Association have published an extensive review of the process and methodology for evaluating cardiovascular research and writing practice guidelines for clinicians.
Cardiome submits Brinavess reimbursement dossier to Belgian authorities
Cardiome Pharma has announced submission of the Brinavess reimbursement dossier to Belgium's national compulsory health care and benefits insurance authority, The National Institute for Health and Disability Insurance.
The incidence of sudden death in heart transplant patients has not...
A first, large multicentre study in the United States has found that despite the advances in clinical management strategies after heart transplantation, there has not been an improvement in the incidence of sudden cardiac death (SCD) in heart transplant patients over the last three decades.
Is antiarrhythmic gene therapy the future of atrial fibrillation management?
Dierk Thomas (Heidelberg, Germany) writes on the results of preclinical research on antiarrhythmic gene therapy.
Cardioxyl Pharmaceuticals completes first clinical trial for heart failure drug CXL-1427
Cardioxyl Pharmaceuticals has announced the positive results of a clinical trial demonstrating that CXL-1427, a novel potential treatment for acute decompensated heart failure (ADHF), was well tolerated in healthy volunteers.
Running five minutes daily can reduce risk of cardiovascular disease related...
Running for only a few minutes a day or at slow speeds may significantly reduce a person's risk of death from cardiovascular disease compared to someone who does not run, according to a study published in the Journal of the American College of Cardiology.
Cook Medical reintroduces Evolution RL lead extraction products to US market
Effective immediately, Cook Medical customers in the United States will again have access to its Evolution RL and Evolution Shortie RL controlled-rotation dilator sheath sets.
St Jude Medical announces CE mark approval and first use of...
The device has been designed to reduce complications associated with ablation procedures through its ability to bend and conform to the cardiac anatomy, decreasing the amount of pressure distributed to a patient's heart wall while simultaneously increasing the stability of therapy delivery.
Niobe system to be presented at Japan Heart Rhythm meeting
The three-day event is the first large-scale opportunity for the Stereotaxis' in-country distributors to promote the clinical value of the Niobe magnetic navigation system for cardiac ablations to the Japan electrophysiology market.
Moderate alcohol use associated with increased risk for atrial fibrillation
Even in moderation, consumption of wine and hard liquor may be a risk factor for atrial fibrillation, according to new research recently published in the Journal of the American College of Cardiology. The research did not identify a similar risk for moderate consumption of beer.
High self-competence may act as a protective factor against lower quality...
A low sense of self-competence seems to contribute to decreased quality of life for paediatric patients with pacemakers, according to results of a study recently published in the Journal of Developmental & Behavioral Pediatrics.
Physicians implant world’s first ProMRI Quadripolar CRT-D system to treat heart...
With Sentus quadripolar leads and the Inventra series, Biotronik offers the only devices for patients with heart failure worldwide that are approved for MRI use.
What is the best strategy for managing atrial fibrillation?
John D Day, president-elect of the Heart Rhythm Society writes that many cases of atrial fibrillation in the USA could be prevented or reversed with lifestyles focussed on health.
Nick Linker
Nick Linker is president-elect of the British Heart Rhythm Society (BHRS) and programme director of Heart Rhythm Congress (HRC). He tells Cardiac Rhythm News about his leadership in the re-design of the BHRS certification process, his goals for his presidential tenure, the key aspects of running a successful electrophysiology department and the highlights of this year's HRC.
More than five million treated in AHA quality improvement programme
The American Heart Association/American Stroke Association's Get With the Guidelines quality improvement programme reached two milestones this month, touching the lives of more than five million patients, including more than three million people who had strokes.
First US patients undergo full-body MRI scans with Biotronik DX system...
Biotronik has announced that the first device patients to undergo full-body magnetic resonance imaging (MRI) scans in the United States have been implanted with the Biotronik DX system.
New telemedicine solution collects heartbeat data wirelessly
GlobalMed introduces the TotalECG, a battery-powered device that streams 12-Lead data securely and wirelessly to interpretation software on a computer that could be up to 100 feet away.
Boston Scientific welcomes new guidance on the use of cardiac technologies...
In the updated Technology Appraisal, people with ventricular arrhythmias are eligible for an ICD, which can help prevent cardiac arrest in those who have previously survived a life-threatening arrhythmia. In people with heart failure, CRT can improve life expectancy and quality of life.
Dennis, Antman to lead American Heart Association in 2014-15
The American Heart Association has announced its national officers for the 2014-15 fiscal year, which begins July 1.
Study finds Medtronic insertable cardiac monitors detect AF in stroke patients...
The CRYSTAL AF trial found that continuous cardiac monitoring with the Reveal XT insertable cardiac monitor was superior to standard care at detecting atrial fibrillation (AF) in patients who have had strokes of undetermined causes.
Novel oral anticoagulants an option for patients with atrial fibrillation
Offering patients anticoagulants could help prevent thousands of strokes and premature deaths from atrial fibrillation (AF), according to NICE.
ANSWER study shows SafeR algorithm reduces right ventricular pacing and improves...
The ANSWER study evaluated whether the minimisation of Vp using the SafeR algorithm improves clinical outcomes for dual-chamber pacemaker patients implanted for sinus node disease or for atrio-ventricular block.
Wearable defibrillator registry (WEARIT-II) update
At Cardiostim/EHRA Europace (18-21 June, Nice, France), Ilan Goldenberg presented an update on one-year follow-up results from the prospective WEARIT-II registry of 1,404 patients using the Lifevest wearable cardioverter defibrillator (Zoll) who were enrolled in the USA from August 2011 through December 2013.
Defibrillation testing: End of an era, or pause for thought?
The need to perform defibrillation testing at the time of implantable cardioverter defibrillator (ICD) surgery has been the focus of significant discussion and debate over the past years. A number of retrospective studies, prospective series, and recently, the results of the long-awaited randomised SIMPLE trial have further suggested that this may be an unnecessary procedure, Jeanne E Poole writes for Cardiac Rhythm News.
Preliminary positive data for Micra presented at Cardiostim/EHRA Europace
Preliminary results from the first four human procedures with the Micra Transcatheter Pacing System (Medtronic) have shown the device has been successfully implanted with no major complications post-implant.
New study of Pradaxa to explore anticoagulation in ablation of AF...
The RE-CIRCUIT study will investigate the safety and efficacy of uninterrupted anticoagulant treatment with Pradaxa in patients with atrial fibrillation who undergo ablation. Results of the study are expected in 2016.
Nanostim leadless pacemaker receives Cardiostim Innovation Award
The award, which was chosen by a congress committee of electrophysiologists and conveys the support of the electrophysiology community, recognises the Nanostim leadless pacemaker as the industry's most innovative product in the sector of electrophysiology and cardiac techniques.
CRT-D devices with AdaptivCRT associated with reduction of costs of AF
New data from the Medtronic Adaptive CRT trial show a 61% (p=0.01) lower risk of atrial fibrillation (AF)-related problems in patients who receive a cardiac resynchronisation therapy-defibrillator (CRT-D) with the Medtronic-exclusive AdaptivCRT algorithm compared to conventional biventricular pacing therapy.
Schwarzer Cardiotek completes integration
Schwarzer Cardiotek has announced that it has completed the integration of the two companies. For the first time, the company showcased the combined portfolio of the merged entity and introduced its new corporate identity at Cardiostim/EHRA Europace (18-21 June, Nice, France).
NICE no longer recommends aspirin alone for reducing risk of stroke...
Updated National Institute for Health and Care Excellence (NICE) guidelines no longer recommend aspirin use alone, solely to reduce the risk of stroke in patients with atrial fibrillation (AF).
Processed red meat linked to higher risk of heart failure, death...
Men who eat moderate amounts of processed red meat may have an increased risk of incidence and death from heart failure, according to a study in Circulation: Heart Failure, an American Heart Association journal.
Population-based study confirms radiofrequency catheter ablation as safe and effective in...
A new population-based study confirms previous study reports that radiofrequency catheter ablation is a safe method of arrhythmia treatment in children with long-term cumulative efficacy exceeding 90%, and a highly significant decrease in the procedure and fluoroscopy time during the study period.
First Latin American registry of catheter ablation of cardiac arrhythmias
Luis Aguinaga and Roberto Keegan give an overview on the design and initial results of the first Latin American catheter ablation of cardiac arrhythmias registry led by the Latin American Society of Electrophysiology and Cardiac Stimulation (SOLAECE).
Sensei X robotic catheter system to be featured at Cardiostim
The Sensei X2 features faster image processing and a slimmer design with the stability and reachability of its predecessor. The improved processing and imaging capabilities of the Sensei X2 will also be available to users of the current Sensei X upon release.
Stereotaxis submits 510(k) application to FDA for Vdrive with V-CAS
Stereotaxis has announced that it has submitted a 510(k) Premarket Notification to the US Food and Drug Administration (FDA) for the company's Vdrive robotic navigation system with V-CAS catheter advancement system.
Greater surveillance necessary to improve significant public health burden of sudden...
New research in the USA finds that the societal burden of sudden cardiac death is high relative to other major causes of death.
First patient enrolled in Stroke Feasibility Study
The Stroke Feasibility Study is the first US clinical study to evaluate the safety of a novel epicardial-based left atrial appendage closure device for stroke prevention in atrial fibrillation patients.
Preventive placement of ICDs in patients with less severe heart failure...
An examination of the benefit of preventive placement of implantable cardioverter-defibrillators (ICDs) in patients with a less severe level of heart failure finds significantly better survival at three years than that of similar patients with no ICD, according to a study in the June 4 issue of JAMA.
eCardio and Yocaly agree to jointly advance remote cardiac monitoring
eCardio Diagnostics and Shandong Yocaly Information Science & Technology, headquartered in Jinan, China, have recently signed a strategic cooperation agreement focused on furthering the advancement of remote cardiac monitoring products and services in the United States, China and beyond.
CE mark for Advisa and Ensura SR MRI SureScan pacemaker systems
Medtronic has announced CE mark approval and commercial launch of the Advisa and Ensura SR MRI SureScan single-chamber pacemaker devices in Europe. Both pacemakers are approved for magnetic resonance imaging (MRI) scans positioned on any region of the body.
EPIC Alliance questions sports ban for young ICD patients
In her presentation at the German Cardiac Society's annual conference, Karin Nentwich (Bad Neustadt, Germany) concluded that implantable defibrillators are not contraindicated for young people engaging in physical education, sports and-within limits-competitive sports.
FDA approves CardioMEMS heart failure system
The US Food and Drug Administration has approved the CardioMEMS heart failure system that measures the pulmonary artery pressures and heart rates of patients with New York Heart Association (NYHA) Class III heart failure who have been hospitalised for heart failure in the previous year. The device allows health care professionals to monitor the condition of their patients remotely.
Free heart checks available in more than 100 pharmacies in North...
AliveCor has announced that in partnership with more than 100 pharmacies across North East London, free heart checks are being offered as part of Heart Rhythm Week, an international campaign from 2-6 June 2014. Participating pharmacies can screen for AF using the AliveCor heart monitor. Blood pressure screenings are also being offered to screen for hypertension.
St Jude Medical completes acquisition of CardioMEMS
St Jude Medical has announced it has completed its acquisition of privately held CardioMEMS, developer of the CardioMEMS heart failure system. The acquisition was completed on 30 May 2014.
Spectranetics receives CE mark for TightRail rotating dilator sheath platform
Spectranetics has announced receiving CE mark approval for its TightRail Rotating dilator sheath platform for mechanical cardiac lead extraction procedures.
Biotronik’s defibrillators for 3.0 Tesla and full-body MRI get CE mark...
Biotronik has announced receiving CE mark approval for its ProMRI technology for ultra high field 3.0 tesla (T) and full-body magnetic resonance imaging (MRI) with the standard 1.5 T scan strength.
Antidote for rapid reversal of dabigatran progresses into next stage of...
Boehringer Ingelheim has announced the next step in the clinical development of idarucizumab, the investigational antidote for rapid reversal of dabigatran-induced anticoagulation
iRhythm secures US$17 million in funding led by Novo A/S as...
iRhythm, has announced that it closed a US$17 million Series E financing led by Novo A/S. Norwest Venture Partners, which led the company's Series D financing, also participated in the oversubscribed round
IN-TIME shows equal benefit of home telemonitoring in ICD and CRT-D...
Home telemonitoring is equally effective in ICD and CRT-D patients, a subanalysis of the IN-TIME trial has shown.
Hospital visits for atrial fibrillation rising
An 11-year study shows that hospitalisations for atrial fibrillation jumped by 23% and costs rose by 24%. The rise in atrial fibrillation's accompanying risk factors might account in part for the rise in hospitalisations.
New data show that defibrillators programmed to wait longer to deliver...
Medtronic has announced the results from the first prospective randomised clinical trial to show that Medtronic implantable cardioverter defibrillators (ICDs) can safely extend detection times before triggering therapy in secondary prevention patients.
New expert consensus statement attempts to close the gap on guidance...
The expert consensus statement provides first-of-its-kind guidance on ICD therapy for the management of patient populations who are not well represented in clinical trials and, as a result, not specifically included in existing guidelines.
Study of Latitude remote patient management system demonstrates significant reduction in...
Patients using the Boston Scientific Latitude remote patient management system with wireless telemetry demonstrated significantly lower mortality and fewer hospitalisations than patients with Latitude-compatible devices who were not followed on the system, according to results from the PREDICt-RM study (Patient related determinants of ICD remote monitoring utilisation and outcomes).
MINERVA shows delays of persistent atrial fibrillation progression with advanced pacing...
Results from the MINERVA (Minimize right ventricular pacing to prevent atrial fibrillation and heart failure) study have found that the Reactive ATP algorithm from Medtronic reduced the development of persistent atrial fibrillation by a 58% relative reduction compared to standard pacemakers (p<0.001).
FDA approves Biotronik Entovis pacemaker system with ProMRI technology
The Entovis system allows patients to undergo Magnetic Resonance Imaging (MRI) scans with a limited exclusion zone. FDA approval covers both single chamber (SR-T) and dual chamber (DR-T) Entovis pacemakers when implanted with Setrox pacing leads.
Cryoballoon faster and more effective than conventional radiofrequency ablation for paroxysmal...
A randomised controlled trial comparing three methods of pulmonary vein isolation for paroxysmal atrial fibrillation has revealed that procedures were faster and more effective with Medtronic's Arctic Front cryoballoon than wide encirclement using conventional point-by-point radiofrequency ablation. Procedures using the cryoballoon also resulted in a higher single procedure success rate.
Aggressive lifestyle management helps improve success rate after AF ablation
Researchers in Australia have found that aggressive management of cardiovascular risk factors and weight can dramatically impact the long-term success rate of freedom from atrial fibrillation following ablation.
Defibrillation testing: Safe but not necessary
Results from the SIMPLE study show that despite the fact that defibrillation threshold testing at the time of device implantation is generally safe, it does not improve implantable cardioverter defibrillator (ICD) shock efficacy or reduce mortality compared to a no-testing strategy.
Substrate based ablation superior than conventional ablation of stable clinical ventricular...
For stable clinical ventricular tachycardias, a substrate based ablation approach is more effective than conventional ablation preventing recurrences from any ventricular tachycardias in patients with ischaemic cardiomyopathy, according to results of the VISTA study, which is the first randomised trial performed in this area.
Gilead announces results from phase 2 study showing reduction in AF...
Data presented at the Heart Rhythm Society Annual Meeting support plans for phase 3 development of a fixed-dose combination of ranolazine and low-dose dronedarone in atrial fibrillation patients.
Study results show significant overall cost savings with St Jude Medical...
St Jude Medical announced that data presented during the 35th Heart Rhythm Society's Annual Scientific Sessions (7-10 May, San Francisco, USA), found that the use of quadripolar leads reduced the number of hospitalisations by 53% when compared to the non-quadripolar group. This hospitalisation rate reduction translated into a statistically significant 62% reduction in overall costs for both health care systems and patients.
New registry data highlight substantial global differences in stroke prevention for...
GLORIA-AF registry programme shows half of patients in China with an irregular heartbeat suboptimally protected against potentially devastating stroke.
TOCCASTAR trial meets primary safety and efficacy endpoints at Heart Rhythm...
Results from the first prospective, randomised study of contact-force ablation technology for the treatment of paroxysmal atrial fibrillation met primary endpoints and supplement the growing body of evidence that supports the safety and effectiveness of contact-force ablation technology.
Richard Fogel
Richard Fogel, president-elect of the Heart Rhythm Society and chief executive officer at St Vincent Medical Group, considers he entered the field of electrophysiology at "the perfect time" when radiofrequency ablation and implantable cardiac defibrillators were emerging as a therapy to treat cardiac rhythm disorders. He spoke with Cardiac Rhythm News about this work, his interests in research and policy and the highlights of this year's Heart Rhythm Society's scientific sessions.
Bariatric surgery prevents obese patients from developing atrial fibrillation
A first-of-its-kind study has found that bariatric surgery helps to prevent atrial fibrillation in patients with morbid obesity. The study was presented at the Heart Rhythm Society's 35th Annual Scientific Sessions (7-10 May, San Francisco, USA).
Spectranetics TightRail rotating dilator sheath achieves clinical goal in first in-patient...
Spectranetics has announced successful completion of the first live case using the TightRail rotating dilator sheath performed by Charles Love. The procedure, according to the company, represents a significant milestone as Spectranetics readies the TightRail tool for full launch later this month.
Updated advice on heart devices from NICE
In final draft guidance, the National Institute for Health and Care Excellence (NICE) has clearly defined which implantable cardiac devices are the most clinically and cost effective. The fresh guidance will help people with a variety of heart conditions including those with an irregular heartbeat.
Eliquis (apixaban) recommended for prevention of stroke
Eliquis (apixaban) has been recommended for continued use for prevention of stroke / systemic embolism in patients with non valvular atrial fibrillation while undergoing cardioversion by European Medicines Agency's Committee for Medicinal Products for Human Use.
St Jude Medical presents comprehensive clinical evidence during Heart Rhythm 2014
Clinical evidence about the company's cardiac rhythm management and ablation technologies will be provided in 38 sessions, including three late-breaking clinical trial sessions.
Boston Scientific joins Optum Labs as Founding Medical Device Partner
Boston Scientific Corporation has joined Optum Labs as the Founding Medical Device Partner to help pioneer new research into effective treatments for heart failure and related cardiac conditions.
CardioFocus’ HeartLight system to be featured at two upcoming arrhythmia conferences
CardioFocus has announced that recent data will be presented at two major conferences focusing on cardiac rhythm management, the 2014 Stanford Biodesign New Arrhythmia Technologies Retreat, and 2014 Heart Rhythm Society (HRS) Scientific Sessions.
Spectranetics debuts next-generation mechanical extraction devices at Heart Rhythm 2014
At the Heart Rhythm Society's 35th Annual Scientific Sessions, the Spectranetics Corporation will focus on management of cardiac device infections and debut of the company's first mechanical lead extraction devices.
Cardiac Lead Extraction Celebrates 25-Year Anniversary
A quarter century ago, doctors treating patients with implanted cardiac pacemakers had a big problem. Their patients were outliving the complex electrical devices that gave them an acceptable quality of life.
Viva cardiac resynchronisation therapy-pacemaker now available in Europe
Medtronic has announced CE mark receipt and the European launch of its newest cardiac resynchronisation therapy-pacemaker, Viva CRT-P.
FDA approves Inspire upper airway stimulation therapy for obstructive sleep apnoea
Inspire therapy is a fully implanted neurostimulation device, the first of its kind for sleep apnoea, that provides an alternative treatment to continuous positive airway pressure that is proven, convenient and easy to use.
Genetics in cardiac arrhythmias wins second and third best oral abstracts...
Researchers studying the genetic aspects of cardiac arrhythmias have won second and third prizes for the best oral abstracts at the 10th Annual Congress of the European Cardiac Arrhythmia Society meeting in Munich, Germany.
Towards resolving the role of M-cells in electrophysiology
At the 10th annual congress of the European Cardiac Arrhythmia Society (ECAS; 23-25 March, Munich, Germany), Fu Siong Ng (London, UK) and colleagues won the first prize for best oral abstract with their research on the role of M-cells in electrophysiology. Ng writes for Cardiac Rhythm News an overview of the subject and the results of their own research.
First reduced radiation heart procedures in Arizona with MediGuide technology
John C Lincoln North Mountain Hospital in Phoenix, Arizona, USA, is the first hospital in Arizona, and only one of eight hospitals in the USA, utilising the MediGuide technology to reduce or eliminate radiation for minimally invasive cardiac procedures, according to a press release from John C Lincoln Health Network.
Advanced Cardiac Therapeutics announces new financing for TempaSure cardiac ablation catheter...
The financing, which was led by New Enterprise Associates and supported by existing investor, NBGI Ventures, will enable the company to continue to advance its next generation TempaSure ablation catheters and related clinical development programme.
New guidelines aim to improve care for unborn babies with heart...
Foetal heart experts working with the American Heart Association have developed guidelines to help healthcare providers care for unborn babies with heart problems, as well as their families.
New approach to cardiac resynchronisation therapy: CRT without left ventricular lead
There are many risks and pitfalls with the current cardiac resynchronisation therapy devices which underscore the need for innovative approaches. Christopher V DeSimone and Samuel J Asirvatham, Mayo Clinic, Rochester, USA, write about a novel pacing lead (developed at Mayo Clinic by Asirvatham, Paul A Friedman, and Charles J Bruce) to help optimise pacing and synchronisation capabilities.
Antiarrhythmic drug therapy increases hospitalisation risk for older patients with atrial...
A single centre analysis of antiarrhythmic drug therapy in older patients with atrial fibrillation and coronary artery disease has found it is associated with increased rehospitalisation at one year, and recommends safer and more effective therapies for symptom control in this group.
Mechanical transvenous extraction of ICD leads with a multiple venous entry-site...
Physicians reporting 15 years of experience in extracting implantable cardioverter defibrillator (ICD) leads with a mechanical single-sheath technique and a multiple venous entry-site approach say they have found it is a complex but safe procedure, with a 99% success rate and no major complications.
First patient enrolled in US pivotal clinical trial to evaluate the...
Medtronic has announced the first US implant of the Evera MRI SureScan implantable cardioverter defibrillator (ICD) System, following US Food and Drug Administration (FDA) approval for its investigational device exemption application and pivotal clinical trial protocol.
Boston Scientific begins NAVIGATE X4 clinical trial
Boston Scientific has announced that it has conducted the first implant in the NAVIGATE X4 clinical trial of the next generation Acuity X4 left-ventricular (LV) pacing leads and Reliance 4-Front defibrillation (ICD) leads.
Symposium features HeartLight Endoscopic Ablation System at 80th Annual German Cardiac...
CardioFocus has announced that leading cardiologists across Germany will gather to discuss clinical experiences with the HeartLight Endoscopic Ablation System for the treatment of atrial fibrillation on 23 April as part of the programme for the 80th Annual Meeting of the German Cardiac Society in Mannheim, Germany.
Boston Scientific announces FDA approval of new defibrillators and heart failure...
Boston Scientific has received FDA approval for its latest generation of defibrillators and heart failure devices. The newly approved devices include the Dynagen Mini and Inogen Mini ICDs, as well as the Dynagen X4 and Inogen X4 CRT-Ds.
AliveCor announces results of SEARCH-AF trial
The SEARCH-AF study conducted in Australia screened 1,000 patients over 65 years old for an abnormal heart rhythm, atrial fibrillation, in 10 community pharmacies in suburban Sydney, Australia. The AliveCor heart monitor was used to capture 30-60 second electrocardiogram (ECG) recordings and wirelessly transmit the recordings to study cardiologists.
CE mark approval for Medtronic Evera MRI ICD system
Medtronic has announced CE mark approval and the European launch of the Evera magnetic resonance imaging (MRI) SureScan implantable cardioverter-defibrillator (ICD) system, the first and only ICD system approved for magnetic resonance imaging scans positioned on any region of the body.
FDA clears mechanical lead extraction devices
Spectranetics announced US Food and Drug Administration (FDA) clearance of two new mechanical lead extraction platforms - the TightRail rotating mechanical sheath and the SlightRail manual dilator sheath - that expand physicians' options for safe removal of cardiac leads.
FDA approves expanded indication for certain pacemakers and defibrillators used to...
The US Food and Drug Administration (FDA) approved an application from Medtronic for revised labelling for two cardiac resynchronisation pacemakers (CRT-P) and eight cardiac resynchronisation defibrillators (CRT-D), expanding the indication for use to patients with atrioventricular (AV) block and less severe heart failure.
Providence Saint Joseph Medical Center announces first patient in US to...
Providence Saint Joseph Medical Center is the first hospital in the USA to conduct a magnetic resonance imaging (MRI) scan of a patient implanted with a new MRI-compatible pacemaker.
Daiichi Sankyo extends the PREFER in AF patient registry
The extension of the registry will include a specific focus on the use of novel oral anticoagulant therapy including prescribing patterns, providing new insight into the long-term management of patients with atrial fibrillation.
Boston Scientific begins expansion of S-ICD system in Asia
Boston Scientific Corporation has expanded the launch of its S-ICD system into parts of Asia. The first implant of the S-ICD system in Asia was performed in Hong Kong by Hung Fat Tse, professor of cardiovascular medicine, The University of Hong Kong and Department of Medicine, Queen Mary Hospital in Pokfulam, Hong Kong, under the proctorship of Martin Stiles, director of Electrophysiology, Waikato Hospital in Hamilton, New Zealand.
First patients implanted with ICDs in Biotronik ProMRI study
Biotronik announced that the first patients across the United States have received implants of the new Iforia implantable cardioverter-defibrillators (ICDs).
Poor sleep doubles hospitalisations in heart failure patients
Poor sleep doubles hospitalisations in heart failure, according to new research in nearly 500 patients presented at EuroHeartCare (4-5 April, Stavanger, Norway).
Brugada-ECG highly prevalent among schizophrenic patients
A study published ahead-of-print in Circulation Arrhythmia and Electrophysiology has found that Brugada syndrome ECG is more prevalent among patients with schizophrenia than patients without schizophrenia. Use of sodium-channel blocking antipsychotics is not attributable to this prevalence.
New data illustrate long-term safety and effectiveness of Boston Scientific S-ICD...
The interim analysis of the ongoing EFFORTLESS S-ICD registry, which evaluated 456 patients with a mean follow-up of 558 days, is the first real-world study to date and confirms the clinical benefits of the S-ICD System in a broad range of patients at risk of sudden cardiac arrest.
Daiichi Sankyo initiates phase 3 ENSURE-AF study
The first patient has been enrolled in the largest planned phase 3 study evaluating novel oral anticoagulant edoxaban in non-valvular atrial fibrillation patients undergoing electrical cardioversion.
Stereotaxis submits 510(k) application to FDA for VDrive with v-loop system
Stereotaxis has announced that it has submitted a 510(k) Premarket Notification with the US Food and Drug Administration (FDA) for the company's Vdrive robotic navigation system with V-Loop variable loop catheter manipulator.
New atrial fibrillation Guideline covers novel anticoagulants, ablation
The 2014 Guideline for the Management of Patients With Atrial Fibrillation includes recommendations for an increased use of radio frequency ablation in the treatment of non-valvular atrial fibrillation, the addition of three new anticoagulants to treatment options, a diminished role in the use of aspirin, and a more comprehensive risk calculator.
New study demonstrates clinical utility of iRhythm’s ZIO service to rule...
iRhythm Technologies announced new study findings that demonstrate the clinical utility of the ZIO Service for ruling in and ruling out cardiac arrhythmias in patients suspected of having the condition, following their discharge from the hospital emergency department.
Long-term data show lower mortality rates in heart failure patients with...
In the longest follow-up to date of cardiac resynchronisation therapy (CRT) for mild heart failure patients, Boston Scientific's multicentre automatic defibrillator implantation trial - cardiac resynchronisation therapy (MADIT-CRT) study demonstrated significant and sustained survival benefit for the indicated population.
CE mark for pacemaker series with event-triggered IEGM transmissions
Biotronik announced CE mark approval for its new Eluna pacemaker series. The new generation of pacemakers includes single and dual-chamber as well as cardiac resynchronisation (CRT-P) devices. The first implantations are currently taking place worldwide.
Remote monitoring of ICD patients improves care
New results from a sub-analysis of the TRUST trial, published in the European Heart Journal, show that remote monitoring of implantable cardioverter defibrillator (ICD) patients improves care and strengthens patient-physician links.
Results from LEADLESS trial published
Leadless pacing has shown to be safe and feasible with the Nanostim (St Jude Medical), self-contained, single-chamber leadless cardiac pacemaker, according to the LEADLESS trial results, recently published in Circulation.
St Jude Medical announces global launch of Optisure defibrillation lead
St Jude Medical announced the global launch of the Optisure defibrillation lead. The Optisure lead features additional Optim insulation at the proximal end of the lead, including under the superior vena cava shock coil.
FDA approves new pacemakers from St Jude Medical
The US Food and Drug Administration (FDA) has approved the Allure Quadra cardiac resynchronisation therapy pacemaker (CRT-P), and the Assurity pacemaker and Endurity pacemaker families of devices.
Edoxaban evaluated in two subgroup analyses of East Asian patients from...
Subgroup analyses of East Asian populations from two phase 3 studies, ENGAGE AF-TIMI 48 and Hokusai-VTE, showed consistent results compared to the global study populations from these trials.
ICD-CRT therapy reduces hospitalisations compared with ICD therapy alone in heart...
A subanalysis within the RAFT trial has shown significantly reduced hospitalisations with implantable cardioverter defibrillators with cardiac resynchronisation therapy (ICD-CRT) devices compared with ICD alone devices in heart failure patients.
Apixaban is cost-effective compared with standard of care for stroke prevention...
Based on randomised trial data and from the UK payer perspective, apixaban is a cost-effective alternative to warfarin and aspirin for stroke prevention in atrial fibrillation patients.
AliveCor over-the-counter orders begin shipping
Over-the-counter orders for the AliveCor Heart Monitor, the only US Food and Drug Administration (FDA) cleared mobile ECG recorder that supports both iPhone and Android smartphones, will now begin shipping.
European post-approval trial for Nanostim leadless pacemaker begins
The purpose of the large European clinical trial is to continue building evidence supporting the safety profile and performance of Nanostim.
Sorin Group acquires Oscor’s CRM lead business
Sorin Group has announced the purchase of Oscor Inc lead business, including a lead manufacturing facility in the Dominican Republic for an aggregate value of approximately US$20 million (€15.4 million).
FDA approves first ICD to be evaluated in US clinical trials...
Biotronik has announced that the Food and Drug Administration (FDA) has approved the expansion of the company's ongoing ProMRI trial. The new phase of the trial (Phase C) will study Biotronik's ProMRI technology in implantable cardioverter defibrillator (ICD) devices.
Insights into investigator sponsored research
Ilan Goldenberg has coordinated investigator sponsored studies in the evaluation of cardiac rhythm devices in Israel. He chaired a session on investigator sponsored research at the 12th International Dead Sea Symposium (IDSS) on Innovations in Cardiac Arrhythmias and Device Therapy (Tel Aviv, Israel, 3-5 March) and spoke to Cardiac Rhythm News about the subject.
National campaign launched in USA to help increase diversity in clinical...
The "I'm In" campaign will help to educate underrepresented populations about clinical trial participation in USA.
Boston Scientific announces CE mark approval and first implants of Ingevity...
The Ingevity family offers a comprehensive set of leads that can be placed using a 6 French introducer, including passive and active fixation models. Ingevity MRI pacing leads are part of the ImageReady MR-conditional pacemaker system.
Novel insights on sudden cardiac death in children
Until now, knowledge on sudden cardiac death in children (1-18 years of age) has been sparse. Bo Gregers Winkel and Jacob Tfelt-Hansen write on the results of a retrospective study that looked at this subject in Denmark.
New onset atrial fibrillation after TAVI does not increase rate of...
A new study has found that new onset atrial fibrillation after transcatheter aortic valve implantation (TAVI) does not increase significantly the rate of stroke or mortality at 30-days and one-year follow-up.
10,000 patients with atrial fibrillation now enrolled in GLORIA-AF registry
The GLORIA-AF registry is expected to provide meaningful knowledge on the global role and use of antithrombotic treatments in protecting patients with atrial fibrillation from the devastating effects of stroke.
Pradaxa (dabigatran etexilate) now approved in more than 100 countries
More than 100 countries have now approved Boehringer Ingelheim's Pradaxa for the prevention of stroke and systemic embolism for adult patients with non-valvular atrial fibrillation.
Outbursts of anger linked to greater risk of heart attacks and...
Outbursts of anger may trigger heart attacks, strokes and other cardiovascular problems in the two hours immediately afterwards, according to the first study to systematically evaluate previous research into the link between the extreme emotion and all cardiovascular outcomes.
First 4F MR conditional lead gets CE mark approval
Biotronik has announced CE mark approval and the first implantations of the Sentus ProMRI lead. Biotronik's bipolar cardiac resynchronisation therapy lead is the first MR conditional lead with a 4 french diameter-approximately 1.6 millimetres.
FDA approves Thermocool SmartTouch catheter
The first catheter ablation therapy in the US to feature direct contact force technology for treatment of atrial fibrillation, has been approved by the US Food and Drug Administration.
Stereotaxis signs distribution agreement for Niobe technology in Japan
Under the agreement, Hokushin Medical will market, sell and distribute the Niobe Magnetic Navigation system and disposable devices to Japanese customers, as well as provide customer training and clinical support.
Scripps Clinic first to implant miniture cardiac monitor
Scripps Green Hospital has become the first hospital in the United States to implant the Reveal Linq insertable cardiac monitor.
Subanalysis of phase 3 ARISTOTLE shows apixaban reduced risk of stroke...
The subanalysis shows that apixaban was more effective than warfarin in reducing the risk of stroke or systemic embolism, was associated with less major bleeding, less total bleeding and less intracranial haemorrhage, regardless of age and in reducing all-cause mortality across age groups.
AliveCor offers integration with Practice Fusion’s electronic health records platform
The partnership between AliveCor and Practice Fusion enables customers of free, web-based electronic health records to seamlessly import AliveCor System's electrocardiogram readings.
Medtronic announces first US implant of world’s smallest, minimally invasive cardiac...
Medtronic announced the first US implant of the Micra transcatheter pacing system, which is one-tenth the size of a conventional pacemaker, and comparable in size to a large vitamin.
Insertable loop recorders offer “the best opportunity” to diagnose unexplained syncope...
Nick Linker, Middlesbrough, UK, recently implanted, for the first time in the UK, the smallest version of an insertable loop recorder. He spoke to Cardiac Rhythm News on the role and advantages of implantable loop recorders in the diagnosis of syncope and cardiac arrhythmias, the main features of this new device and its cost-effectiveness.
Detection of atrial fibrillation with insertable cardiac monitors is superior to...
Results from the CRYSTAL AF trial have shown that atrial fibrillation in patients with cryptogenic stroke is better detected with insertable cardiac monitors than standard monitoring at six, 12 and 36 months.
Reveal Linq insertable cardiac monitor receives FDA clearance and CE mark
Medtronic has announced US Food and Drug Administration (FDA) 510(k) clearance, CE mark, and the global launch of its Reveal Linq insertable cardiac monitor system, a small, wireless monitor that provides long-term remote monitoring to help physicians diagnose and monitor irregular heartbeats.
Limitations of novel oral anticoagulants challenge their widespread use
Even though novel oral anticoagulants present an "amazing opportunity to improve stroke prevention in atrial fibrillation", according to Peter Kowey (Philadelphia, USA), there are still several limitations with regards to monitoring, discontinuation, lack of reversal agents and lack of long-term data that affect their widespread use.
Apama Medical announces claim coverage in China for electrode-based ablation balloon...
Apama Medical announced receipt of a Chinese notice of allowance for claims covering their low profile electrode assembly that enables the attachment of flexible electrodes to an ablation balloon for energy delivery and monitoring.
Stereotaxis appoints new chief executive officer
Stereotaxis announced that its board of directors has appointed Chairman William C Mills III to chief executive officer, effective February 12, 2014. Mills has been serving as interim chief executive officer since April 2013.
New data support use of Zio Patch to identify cardiac arrhythmias...
New study data support the use of the Zio Patch to help identify underlying cardiac arrhythmias in patients who have had a stroke or transient ischaemic attack. The Zio Patch enables long-term continuous monitoring to detect sporadic heart rhythm disturbances, including atrial fibrillation.
New Heart Rhythm Society Choosing Wisely list details five commonly used...
The Heart Rhythm Society developed the list as part of Choosing Wisely, an initiative of the American Board of Internal Medicine Foundation launched in 2012 aimed at curbing the use of certain tests and procedures that are not supported by clinical research.
AtriCure completes acquisition of Estech
AtriCure has announced the completion of the previously publicised acquisition of Endoscopic Technologies. Estech develops and markets temperature controlled radiofrequency energy technologies for the treatment of atrial fibrillation.
Vernakalant demonstrates 74% cardioversion in post-cardiac surgery patients with atrial fibrillation
A poster presented at the 43rd Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery has shown that vernakalant (Brinavess, Cardiome) successfully cardioverted 74% of atrial fibrillation patients to sinus rhythm at 90 minutes.
Insights into BERLIN study of catheter ablation for ventricular tachycardia treatment
The BERLIN study is a prospective, multicentre study that seeks to determine the best time for catheter ablation in the treatment of patients with sustained ventricular tachycardia and coronary artery disease.
FDA clears AliveCor Heart Monitor over-the-counter
AliveCor has announced that the US Food and Drug Administration (FDA) has granted the company over-the-counter clearance for its AliveCor Heart Monitor, a single-channel ECG recorder, previously available by prescription only.
Cardiac resynchronisation therapy with leadless technology is feasible in heart failure...
Heart failure patients treated in the WISE-CRT study with a leadless ultrasound-based device demonstrated a significant improvement of functional status and a significant increase of left ventricular ejection fraction at six-month follow-up.
EPIC Alliance empowers women in electrophysiology
Cardiac Rhythm News spoke to Jeanne Poole, one of the founding members of the steering committee of the EPIC Alliance, about the creation, objectives and key achievements of this organisation that empowers women working or wanting to work in electrophysiology.
First US patient implant with the Nanostim leadless pacemaker
St Jude Medical has announced the first US implant in the company's LEADLESS II pivotal trial designed to evaluate the Nanostim leadless pacemaker for US Food and Drug Administration (FDA) approval.
eCardio opens independent diagnostic testing facility in California
The diagnostic testing facilities provide 24-hour monitoring services for remote cardiac monitoring prescribed by physicians, including mobile cardiac telemetry, cardiac event monitors and holters.
Rome to host ESC Congress for the first time
Rome will be the host of the European Society of Cardiology Congress 2016.
Aspirin still overprescribed for stroke prevention in atrial fibrillation
According to a recent study, aspirin is still overprescribed for stroke prevention in atrial fibrillation despite the potential for dangerous side effects.
Israel Ministry of Health approves clinical trials for BioControl Medical’s CardioFit...
Israel Ministry of Health has approved clinical trials of the CardioFit system in patients with chronic heart failure.
First patients enrolled in Biotronik’s expanded ProMRI study
Biotronik has announced that that the first patients have been enrolled in an expansion of its ongoing ProMRI trial. The aim of the study is to evaluate the safety and effectiveness of the company's pacing systems in patients undergoing full-body MRI.
Leadless pacemaker implanted in the UK for the first time
St Jude Medical has announced the first post-approval implant of its Nanostim leadless pacemaker in the UK. Richard Schilling implanted the pacemaker at St Bartholomew's Hospital in London, UK.
FDA approves Medtronic SureScan Pacing Systems for full body MRI scans...
Patients implanted with the Advisa DR MRI or Revo MRI SureScan pacing systems now can have MRI scans without positioning restrictions, including the chest area, which previously had been restricted.
New combined mapping and ablation system for atrial fibrillation treatment undergoes...
Hans Kottkamp, Zurich, Switzerland, presented a new device strategy that combines diagnostic and treatment capabilities for atrial fibrillation in one system, at the AF Symposium in Orlando USA (9-11 January 2014). He spoke to Cardiac Rhythm News about the combined system.
NICE consults on draft updated recommendations on implantable devices for the...
The draft guidance from the UK's National Institute for Health and Care Excellence (NICE) defines which implantable cardiac devices are clinically and cost effective options for people with life-threatening arrhythmias or heart failure.
Ablation of persistent atrial fibrillation, are we there yet?
Mark Earley (London, UK) argues that despite the breathtaking progress of interventional electrophysiology in the last 10 years, ablation of persistent atrial fibrillation remains an unconquered summit.
Anne Curtis
Anne Curtis is past president (2005-2006) of the Heart Rhythm Society. She has been involved in various clinical trials in electrophysiology including her most recent trial, BLOCK HF, and is currently a member of different editorial boards in the field. She spoke to Cardiac Rhythm News about her career achievements, memorable cases and the major challenges executing a trial.
Therapy Cool Flex Ablation Catheter and Cardiac Ablation Generator get FDA...
The FDA has approved St Jude Medical's Therapy Cool Flex Ablation Catheter and Cardiac Ablation Generator for the treatment of atrial flutter.
Remote follow-up of patients with implantable defibrillators significantly reduces ambulatory costs
New data from the ECOST study show remote monitoring with Biotronik Home Monitoring significantly reduces the cost of follow-up in patients with implantable defibrillators (ICDs).
HeartLight endoscopic ablation system can now be used in Spain
CardioFocus has announced that its HeartLight endoscopic ablation system is now available in Spain and Hospital Universitari i Politecnic La Fe in Valencia was the first in the country to use the technology.
Biotronik now supplies US Department of Veterans Affairs
Biotronik has announced that its devices and leads, including the latest DX system technology, has been added to the list of cardiac rhythm management device suppliers to the US Department of Veterans Affairs.
Stereotaxis completes clinical trial of Vdrive with V-Loop system
Results of the 120-patient study, which was conducted at three sites in the USA and two in Europe, will be included in a 510(k) premarket notification the company intends to submit in the first quarter of 2014.
Medtronic announces acquisition of Tyrx
Tyrx's product offerings include the recently FDA cleared Aigisrx R Fully Resorbable Antibacterial Envelope, designed to reduce surgical site infections associated with cardiac implantable electronic devices, and the Aigisrx N Antibacterial Envelope, for use with spinal cord neuromostimulators.
VICTORY AF trial for persistent or long-standing atrial fibrillation starts enrolment
The study will evaluate the safety of the Phased Radiofrequency system (Medtronic) for the treatment o patients with persistent or long-standing atrial fibrillation.
Topera’s third generation 3D Mapping System is FDA cleared
The new system processes information in seconds, providing near-instantaneous intra-procedural mapping and re-mapping capabilities. In addition, the system incorporates a new color-imaging module to aid identification of "Rotors," an electrophysiologic phenomenon previously shown to sustain atrial fibrillation.
AtriCure completes acquisition of Estech
On 2 January, AtriCure announced the completion of the previously announced acquisition of Endoscopic Technologies (Estech), a developer of surgical ablation devices with controlled radiofrequency technology used in the treatment of atrial fibrillation.
Depression more common in patients with persistent atrial fibrillation than in...
Researchers in Germany have reported that depressed mood tends to be more severe in patients with persistent atrial fibrillation than in patients with paroxysmal atrial fibrillation. The study comparing populations from two large, recent, controlled clinical trials in atrial fibrillation was published ahead-of-print in Europace.
The challenge of optimal ICD programming
Despite new algorithms for better discrimination of supraventricular from ventricular tachyarrhythmias being constantly developed, Helmut U Klein writes that the problem of inappropriate implantable cardioverter defibrillator (ICD) therapy remains unsolved.
Subcutaneous ICD continues to show positive results in a real-world scenario
Updated results of the EFFORTLESS S-ICD registry have shown the Subcutaneous Implantable Cardioverter Defibrillator (S-ICD, Boston Scientific) continues to show effective conversion of induced and ambulatory ventricular tachycardia or fibrillation (VT/VF) comparable to standard ICD performance and low complication rates.
AtriCure to acquire Estech
AtriCure has announced entering into a definitive merger agreement to acquire Estech, a developer of surgical ablation devices with controlled radiofrequency technology used in the treatment of atrial fibrillation.
New atrial fibrillation classification by ECG will help GPs deliver personalised...
A new classification of atrial fibrillation (AF) by electrocardiogram (ECG) is set to be developed by European experts to aid personalised management of condition.
New drug application for edoxaban submitted in Japan
Daiichi Sankyo is seeking approval in Japan for edoxaban in new indications for non-valvular atrial fibrillation and symptomatic venous thromboembolism.
Imperial College awarded research grant to develop new drug treatments for...
The UK charity Heart Research UK has awarded a grant of £125,000 to the National Heart and Lung Institute at Imperial College, London, UK, for research of new drug treatments to target cells that cause cardiac arrhythmias.
Management of atrial fibrillation still suboptimal in Europe
Results of EORP-AF, a pilot registry on the management and treatment of atrial fibrillation in Europe, have shown that compliance with treatment guidelines for atrial fibrillation patients with the lowest and higher stroke risk scores remains suboptimal.
Meta-analysis shows all four novel oral anticoagulants perform better than warfarin
A first-of-its-kind meta-analysis comparing four new oral anticoagulants with warfarin has found they perform better than warfarin reducing stroke, intracranial haemorrhage and mortality in atrial fibrillation patients. The study was published ahead-of-print in The Lancet.
Rivaroxaban prevents prothrombogenic remodelling of human atrial myocardium
Andreas Goette (Paderborn, Germany) explains the results of a study which found how rivaroxaban (Xarelto, Bayer Healthcare) helps to prevent prothrombogenic remodelling of human atrial myocardium.
Next-generation X4 quadripolar CRT-D systems are CE-marked
Boston Scientific has announced it has received CE mark approval of its X4 line of quadripolar CRT-D systems including Autogen X4, Dynagen X4, and Inogen X4 cardiac resynchronisation therapy defibrillators (CRT-Ds), a suite of Acuity X4 quadripolar left ventricular (LV) leads and the Acuity Pro lead delivery system.
Multicentre study finds focal impulse and rotor mapping (FIRM) may eliminate...
Preliminary results of a new multicentre study have found that both paroxysmal and persistent atrial fibrillation may be sustained by localised stable rotors and that targeted ablation of those sources by focal impulse and rotor mapping (FIRM) can eliminate atrial fibrillation
FDA advisory panel votes favourably on Watchman left atrial appendage closure...
On 11 December, the FDA's Circulatory System Devices Panel of the Medical Devices Advisory Committee voted favourably by a majority, Yes: 13, No: 1, that the benefits of the Watchman left atrial appendage closure device outweigh the risks.
Medtronic announces first-in-human implant of Micra Transcatheter Pacing System
On 9 December, Medtronic announced the first-in-human implant of its Micra Transcatheter Pacing System (TPS). According to the company, this is the world's smallest pacemaker and does not require transvenous leads.
More efficient way to grow heart muscle from stem cells could...
Researchers in the USA and Hong Kong have developed a two-step system that helps to differentiate human embryonic stem cells from induced pluripotent stem cells. This process results in high efficiency and reproducibility of heart muscle, which mimics normal cardiovascular development.
FDA issues safety communication on HeartStart automated external defibrillators
Certain HeartStart automated external defibrillator (AED) devices made by Philips Healthcare may be unable to deliver needed defibrillator shock in a cardiac emergency situation, the US Food and Drug Administration (FDA) has announced.
Radiation exposure in the cardiac electrophysiology lab: our responsibility to change
Eugenio Picano, CNR, Institute of Clinical Physiology, Pisa, Italy, writes for Cardiac Rhythm News a summary of the paper: "The appropriate and justified use of medical radiation in cardiovascular imaging. A position document of the ESC associations of cardiovascular imaging, percutaneous cardiovascular interventions and electrophysiology", recently published in the European Heart Journal. He also spoke on the subject at Venice Arrhythmias (27-29 October, Venice, Italy).
GlobalData forecasts Cardiac Rhythm Management market annual growth rate of 4.6%
The global cardiac rhythm management (CRM) market is set to experience significant expansion and emerging market adoption, with revenues climbing from US$10.9 billion in 2012 to more than US$15.7 billion by 2020.
Sorin receives CE mark for its Kora 100, a new MRI...
Sorin has announced the CE mark approval and first implant of the Kora 100 pacing system. The new device enables implanted patients to undergo magnetic resonance imaging (MRI) safely.
Does the anatomy of left atrial appendage correlate with the risk...
Luigi Di Biase writes for Cardiac Rhythm News on the results of a study that identified four different left atrial appendage morphologies and its relationship with the risk of stroke in atrial fibrillation patients. "These results could have a relevant impact on the oral anticoagulation management and occlusion device management of atrial fibrillation patients with an intermediate risk for stroke," writes Di Biase.
Atricure receives FDA approval for investigational study of AtriClip
The study will evaluate the use of the AtriClip left atrial appendage exclusion system to prevent stroke in patients with atrial fibrillation as alternative to anti-coagulation therapy.
Phase IIb study of vanoxerine shows strong safety efficacy signals for...
ChanRx has announced positive safety and statistically significant efficacy data from a phase IIb study of vanoxerine (GBR-12909), a drug in development for the treatment of atrial fibrillation. The data were presented yesterday at the American Heart Association 2013 Scientific Sessions (16-20 November, Dallas, USA).
Late-breaking trial results show significant delay in atrial fibrillation disease progression...
New research shows that pacemakers with enhanced-pacing features (Medtronic) have the ability to slow the progression of atrial fibrillation in patients with bradycardia, or a slow heartbeat.
AFNET-EAST trial enrols 1,000 patients
On 4 November the 1,000th patient was enrolled in the AFNET-EAST trial. The pan-European clinical trial compares two different treatment strategies in atrial fibrillation EAST trial compares early rhythm control therapy to usual care.
AliveCor launches mobile app to monitor heart rhythm
AliveCor has launched a mobile heart health service, offering expert review of electrocardiograms recorded through their smartphone app. Patients can share these expert reviews and their electrocardiograms with their physicians. Physicians in turn can manage their patients through AliveCor.com, using their PC or tablet.
Edoxaban not inferior to warfarin in stroke prevention for atrial fibrillation...
Results from the ENGAGE AF-TIMI 48 clinical trial have found that the investigational, oral, once daily direct factor Xa-inhibitor edoxaban (Lixiana, Daiichi-Sankyo) met the primary efficacy endpoint of non-inferiority compared to warfarin for the prevention of stroke or systemic embolic events in patients with non-valvular atrial fibrillation.
Weight reduction decreases severe atrial fibrillation symptoms
A new study published in the Journal of the American Medical Association has shown that a structured weight reduction programme for highly symptomatic atrial fibrillation patients helps to reduce symptom burden and severity and also helps to reduce antiarrhythmic drug use.
Use of device for chest compressions compared to manual CPR does...
A study presented at the American Heart Association Scientific Sessions (16-20 November, Dallas, USA) has shown that mechanical chest compressions in combination with defibrillation during ongoing compressions provided no improved four-hour survival vs. manual cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest.
Resting pulse rates of UK pre-teens have risen during past 30...
The resting pulse rate of UK pre-teens may have risen by up to two beats a minute during the past 30 years. But the rise does not seem to be linked to the overall weight gain seen in this age group during this period, reveals research published online in the Archives of Disease in Childhood.
CardioInsight secures financing to advance ECVUE mapping system development
CardioInsight has announced that it has completed a long-term strategic financing that provides the company with an initial investment of US$15 million.
Magnetic analogue of ECG shows 89% accuracy diagnosing long QT syndrome...
A first-of-its-kind study has found that foetal magnetocardiography (fMCG)-the magnetic analogue of electrocardiograms (ECG)-may help to diagnose and possibly treat in utero long QT syndrome.
Martek Medical launches Lifeline Pro AED in the UK
Martek Medical has released the Lifeline Pro automated external defibrillator (AED) in the UK market. The Lifeline Pro AED (Defibtech), according to a company release, has the quickest charge to shock time of any defibrillator on the market at just four seconds.
New efficacy and safety data for dabigatran to be announced at...
Boehringer Ingelheim has announced the upcoming presentation of the latest efficacy and safety data for the novel oral anticoagulant dabigatran etexilate (Pradaxa) at the 2013 American Heart Association's (AHA) Scientific Sessions (16-20 November, Dallas, USA).
Radiation exposure in supraventricular tachycardia ablation reduced with minimally fluoroscopic mapping...
Radiofrequency ablation of supraventricular tachycardia guided by the non-fluoroscopic Ensite NavX mapping system (St Jude Medical) has shown lower levels of ionising radiation exposure for the patient and for the operator compared with conventional catheter ablation, according to preliminary results from the NO-PARTY Italian trial.
FDA clears Firmap catheter and RhythmView 3D mapping workstation
On 7 November, Topera announced receiving 510(k) clearance by the FDA for the Firmap catheter and the RhythmView 3D mapping workstation. This technology has been developed for the identification of the sources that sustain complex cardiac arrhythmias.
Boston Scientific completes acquisition of Bard Electrophysiology
On 1 November, Boston Scientific closed on its previously announced agreement to acquire Bard EP, the electrophysiology (EP) business of CR Bard.
First patient enrolled in EWOLUTION registry for Watchman device
EWOLUTION is a multinational, prospective, multicentre, post market registry aimed to collect data on procedural success, incidence of stroke and mortality of patients implanted with a Watchman device.
Resveratrol derivative shows promise in treating atrial fibrillation
A new study published in the British Journal of Pharmacology has shown that a new medication based on resveratrol-a compound found in red wine and nuts-is an effective inhibitor of several potential targets involved in atrial fibrillation development. Cardiac Rhythm News spoke to Peter Light, lead author of the study.
American College of Cardiology announces contest to recognise people living well...
Through its CardioSmart patient initiative, the American College of Cardiology is hosting a contest to find six individuals who are living well with heart disease and can inspire others to take charge of their heart health.
CHART-1 trial for cellular therapy of heart failure to enrol patients...
The CHART-1 trial, according to a company release, represents the world's first phase III trial in regenerative medicine for a pre-programmed cellular therapy targeting heart failure.
Stereotaxis Niobe system receives highest reimbursement classification in Japan
Stereotaxis has announced that the Ministry of Health, Labor and Welfare (MHLW) in Japan has classified its Niobe Magnetic Navigation System as a C2 medical device.
Cardiome announces publication of positive data from an observational, retrospective study...
Cardiome announced on 7 October the publication of positive data from an observational, retrospective study investigating Brinavess for atrial fibrillation. The study was performed at Skane University Hospital in Malmö, Sweden.
NATF launches atrial fibrillation action initiative
The North American Thrombosis Forum (NATF) has launched an innovative educational initiative on risk assessment and anticoagulation for stroke prevention in atrial fibrillation.
Discrepancies in CRT-ICD battery longevity
An independent study demonstrates significant differences in battery longevity between cardiac resynchronisation therapy (CRT) implantable cardioverter defibrillators (ICDs), which are still implanted today worldwide.
Clinical strategies for selecting oral anticoagulants in atrial fibrillation patients
With the advent of new oral anticoagulants, there are many questions towards selection of the most appropriate treatment for patients with atrial fibrillation. Sean D Pokorney (Durham, USA) writes for Cardiac Rhythm News about the different clinical strategies to take into consideration based on the results of landmark clinical trials of novel oral anticoagulants.
HeartLight US pivotal trial completes enrolment
The randomised, controlled trial of the HeartLight Endoscopic Ablation System (Cardiofocus) completed enrolment of over 400 patients from 21 research sites across the United States.
Antonio Raviele
Antonio Raviele (Venice, Italy), cofounder of the VeniceArrhythmias congress, implanted the first intravenous defibrillator lead in Italy. He spoke to Cardiac Rhythm News about this experience, his current research, his projects dedicated to enhance awareness on cardiac arrhythmias and the highlights of VeniceArrhythmias 2013
St Jude Medical announces acquisition of Nanostim and CE mark of...
On 14 October, St Jude Medical announced the completion of its acquisition of Nanostim, a privately-owned developer of miniaturised, leadless pacemakers.
Biotronik launches Idova 7 ICD and CRT-D series
According to a company release, the Idova 7 technology offers ultrahigh-energy therapy without compromising on short charge times (10sec), smaller size (34cc), and longevity of more than 11 years.
Resynchronisation in narrow QRS patients questioned
The results of the EchoCRT study show that cardiac resynchronisation therapy (CRT) does not reduce the rate of death or hospitalisation and may increase mortality in heart failure patients with a QRS duration of less than 130msec
FDA Advisory Panel Recommends expanded indication for Medtronic CRT devices in...
On 8 October, the FDA's Circulatory Systems Devices Advisory Panel voted that biventricular pacing with Medtronic devices is beneficial for treating patients who have atrioventricular block and left ventricular systolic dysfunction, compared to conventional right ventricular pacing.
2010 ESC guidelines on atrial fibrillation followed by seven European countries,...
The guideline-recommended use of oral anticoagulation has increased compared to previous registries, as well as the use of antiarrhythmic drugs and catheter ablation procedures. Data have recently been published in Europace
Latin American health policies adversely affect treatment of cardiac arrhythmias in...
Public health expenditure in Latin America is directed mainly to tackle diseases and social problems that kill the young population but not to treat cardiac diseases (including arrhythmias), which mainly affect older people, writes Diego Vanegas, Bogota, Colombia
FDA approves Biotronik’s Ilesto ICD and CRT-D devices
On 1 October, Biotronik announced the Food and Drug Administration (FDA) approval for its Ilesto family of implantable cardioverter-defibrillator/cardiac resynchronisation therapy defibrillators (ICD/CRT-D).
AtriCure launches Maze IV training programme in Europe
On 1 October, AtriCure launched its Maze IV training programme on surgical ablation for the treatment of atrial fibrillation at the Sana Stuttgart Cardiac Surgery Center in Germany.
Remede implantable device system shows “promising” results in the treatment of...
Patients with central sleep apnoea, treated with the remede system (Respicardia), experienced a 50% decrease in apnoea-hypopnea index, improved oxygenation by over 50%, decreased arousals and improved quality of life, according to results of the remede system pilot study presented by William T Abraham at the 17th Heart Failure Society of America meeting.
Heart failure patients experience 46% reduction in atrial fibrillation risk with...
On 23 September, Medtronic announced clinical trial results showing that heart failure patients treated with its AdaptivCRT feature experienced a 46% reduction in atrial fibrillation risk.
Vernakalant intravenous achieves better conversion to normal sinus rhythm than propafenone...
Patients treated with vernakalant achieved conversion to normal sinus rhythm in a median time of 12 minutes compared to 151 minutes for the propafenone group and 162 minutes for the flecainide group (p<0.01), according to results published in the Journal of Atrial Fibrillation.
HAS-BLED score more accurate than stroke scores at predicting major bleeding...
A new study has identified that, in anticoagulated patients with atrial fibrillation (AF), the HAS-BLED score has better prediction accuracy for major bleeding than the CHADS2 or CHA2DS2-VASc scores.
Biotronik launches AlCath Flutter Gold for atrial flutter treatment
The company announced that Thorsten Lewalter, director of the Isar Heart Center Munich, Medical Center for Cardiology and Internal Intensive Care, Munich, Germany, successfully performed the first AlCath Flutter case worldwide as part of an international product evaluation.
No relationship between seizure and sudden cardiac arrest in most patients...
Seizure in patients with epilepsy may not be a trigger of sudden cardiac arrest (SCA). Eric Stecker, Knight Cardiovascular Institute, Oregon Health and Science University, Portland, USA, and colleagues, from the Cedars-Sinai Heart Institute, Los Angeles, USA, reported in a study recently published in Circulation Arrhythmia and Electrophysiology.
Sports and arrhythmias: to screen or not to screen
Screening athletes for the risk of sudden cardiac arrest has been a topic of discussion for many years. Jacob Tfelt-Hansen, author of a Danish study on this subject, gave an overview about different positions and studies that favour or disfavour this practice.
Boston Scientific opens Institute for Advancing Science and Innovation Center in...
The Institute for Advancing Science will offer training in clinical practice and multidisciplinary programmes in Interventional Cardiology, Cardiac Rhythm Management and Electrophysiology, Endoscopy, Peripheral Interventions and Urology and Women's Health.
Chinese electrophysiology market to grow approximately 20% in 2013
According to research by the Millennium Research Group, the Chinese electrophysiology market will grow approximately 20% in 2013, based on the largest available sampling of ablation procedures.
AtriCure appoints Scott Drake to its board of directors
AtriCure has announced that Scott Drake, chief executive officer of Spectranetics has been elected as director. Donald C Harrison, one of the founders of AtriCure, has also informed the board of directors of his plans to retire at the 2013 Annual Meeting in May 2014.
SERVE-HF trial completes enrolment
SERVE-HF is an international, randomised study of 1,325 participants investigating if the treatment of central sleep apnoea improves survival and outcomes of patients with stable heart failure.
Delayed enhancement-MRI detected atrial fibrosis could predict successful ablation in atrial...
Atrial fibrosis detected using delayed enhancement magnetic resonance imaging (DE-MRI) seems to be an independent predictor of procedural outcome in patients undergoing ablation of atrial fibrillation, according to results of the DECAAF (Delayed enhancement-MRI determinant of successful catheter ablation of atrial fibrillation) study
Catheter ablation may reduce stroke risk in atrial fibrillation patients
A first of its kind study has shown that patients with atrial fibrillation who undergo catheter ablation have a lower stroke risk than patients who do not undergo the procedure independent of CHADS2 score.
Idiopathic ventricular tachycardia: transcatheter ablation or antiarrhythmic drugs?
Claudio Tondo (Milan, Italy) analyses the best treatment options for different kinds of idiopathic ventricular tachycardias. He will be talking about this subject at Venice Arrhythmias (27-29 October, Venice Italy).
WaveCrest LAA Occlusion System is CE-marked
The WaveCrest is an implantable device that seals off the left atrial appendage (LAA) opening so clots cannot escape into the blood stream and cause a stroke.
Out of hospital cardiac arrest survival just 7%
Survival for out-of-hospital cardiac arrest is just 7%, according to research presented at ESC congress 2013 by Xavier Jouven, Hopital Georges Pompidou, Paris, France and Wulfran Bougouin, Paris, France.
Sensor-based electromagnetic tracking system may help to reduce radiation exposure in...
A new technology that uses a sensor-based electromagnetic tracking system may help to guide cardiac resynchronisation therapy (CRT) device implantation safely and potentially cut radiation exposure time as compared with traditional fluoroscopy.
GARFIELD registry shows antithrombotic agents not optimally used to prevent stroke
The findings, from eight abstracts presented this week at the ESC congress 2013, collectively show that anticoagulant therapy which is known to significantly lower stroke risk in AF patients-is consistently under-utilised among those at-risk atrial fibrillation patients.
IN-TIME study shows significant reduction in all-cause mortality in ICD and...
Results from the IN-TIME study have shown a significant reduction in all-cause mortality in heart failure patients with implantable cardioverter defibrillators (ICDs) or cardiac resynchonisation therapy defibrillators (CRT-Ds) fitted with remote monitoring compared to standard care therapy.
Stroke or systemic embolism uncommon in AF patients treated with apixaban...
Results of a post-hoc subanalysis from the phase III ARISTOTLE trial have shown that stroke or systemic embolism and major bleeding were uncommon within 30 days in non-valvular atrial fibrillation patients who undertook a medical procedure.
REFORM study shows Biotronik Home Monitoring reduces follow-up visits in ICD...
The study, according to a company release, demonstrates that implantable cardioverter defibrillator (ICD) patients with Biotronik's Home Monitoring can enjoy a reduction in follow-up visits by 58%. This in turn leads to patients reporting enhanced quality of life.
New trial shows subcutaneous implantable cardioverter defibrillator is safe and effective
A new trial has found that the subcutaneous implantable cardioverter defibrillator (S-ICD System, Cameron Health/Boston Scientific) is safe and effective at detecting and treating both induced and spontaneous ventricular tachycardia and ventricular fibrillation in a broad range of patients requiring implantable cardioverter defibrillators (ICDs).
AtriCure and Century Medical secure a distribution agreement
AtriCure and Century Medical of Japan have announced the extension and expansion of their multi-year agreement in which Century Medical will distribute AtriCure's surgical ablation technologies to hospitals in Japan.
Implantation of the Cognis cardiac resynchronisation therapy defibrillator begins in China
The first patient implants of the Cognis (Boston Scientific) cardiac resynchronisation therapy defibrillator (CRT-D) were successfully performed in China's Zhejiang province and Beijing by Farong Shen in Zhejiang Lucheng Hospital and Wei Hua in Beijing Fuwai Hospital.
Personalised atrial fibrillation management needed to close mortality gap
Personalised management is the only way to close the mortality gap for patients with atrial fibrillation, according to an ESC consensus paper presented at ESC Congress 2013 by Paulus Kirchhof (UK).
AlluraClarity reduces radiation in image-guided catheter ablation trial in The Netherlands
The Catharina Hospital (Eindhoven, The Netherlands) and Royal Philips has announced the results of a clinical study involving the treatment of 136 patients with complex heart rhythm disorders such as atrial fibrillation.
Discrepancies found in antiarrhythmic drug prescriptions for atrial fibrillation
Chern-En Chiang, General Clinical Research Center and Division of Cardiology, Taipei Veterans General Hospital, National Yang-Ming University, Taipei, Taiwan, and colleagues, investigated the appropriate uses of antiarrhythmic drugs in atrial fibrillation patients and reported that there were inconsistencies in drug prescription compared to guidelines issued in 2006.
FDA approval for IntellaTip MiFi XP ablation catheter and 510(k) clearance...
Boston Scientific Corporation has announced it has received US Food and Drug Administration (FDA) approval of its IntellaTip MiFi XP catheter and 510(k) clearance of its Zurpaz 8.5F steerable sheath. The IntellaTip is indicated for ablation of atrial flutter.
Targeting electrical delay and clinical outcome in cardiac resynchronisation therapy patients
A study undertaken by a team at the Massachusetts General Hospital, Boston, USA, suggests there is a need to individualise physicians' pacing approach for patients receiving cardiac resynchronisation therapy. Jagmeet Singh writes about the outcomes of this study.
Valentin Fuster named editor in chief of ACC’s flagship journal
World-renowned cardiologist and researcher Valentin Fuster has been named editor in chief of the Journal of the American College of Cardiology, the flagship journal of the American College of Cardiology.
St Jude Medical acquires Endosense
St Jude Medical has announced the acquisition of Endosense. The acquisition adds to the company's electrophysiology portfolio. St Jude Medical has made an initial payment of approximately 159 million Swiss Francs (US$170 million) and acquired 100% of the outstanding equity of Endosense.
BioVentrix expands use of its Revivent System for heart failure treatment...
BioVentrix has announced the first use of its Revivent Myocardial Anchoring via less invasive ventricular enhancement (the LIVE) procedure in Germany. The successful procedure was performed on a 54-year old man suffering from advanced heart failure at the Schön Klinik Vogtareuth in Vogtareuth, Germany.
FDA approval and first use of MediGuide enabled ablation catheters announced
St Jude Medical announced on 15 August that the US Food and Drug Administration (FDA) has approved its MediGuide enabled ablation catheters. The catheter can be tracked using 3D visualisation on previously recorded fluoroscopic images in real time.
Odds of surviving sudden cardiac arrest increase at fitness facilities
A new study published online in the Journal of the American College of Cardiology has found that people experiencing sudden cardiac arrest at exercise facilities have a higher chance of survival than at other indoor locations, likely due to early cardio-pulmonary resuscitation and access to an automated external defibrillator (AED), among other factors
TYRX announces first US implantation of AIGISRx R fully bioresorbable antibacterial...
TYRX has announced that the first implantation of its new AIGISRx R fully resorbable antibacterial envelope has taken place at the Vanderbilt Heart and Vascular Institute in Nashville, USA, by Christopher R Ellis. The AIGISRx R antibacterial envelope received US Food and Drug Administration (FDA) clearance on 20 May 2013.
Single-chamber ICDs with novel detection algorithms show low inappropriate shock rate
Primary results from the PainFree SST study have shown that 97.6% of patients were free from inappropriate shocks with single-chamber implantable cardioverter defibrillators (ICDs) at one year of implantation.
How to prevent complications of catheter ablation for atrial fibrillation
John D Day (Murray, USA) has performed more than 3,000 catheter ablation for atrial fibrillation procedures. Based on his experience, he describes five basic principles to minimise the risk of serious complications.
First patients enrolled in new study design of ARTISAN-AF trial for...
ARTISAN-AF study is a pivotal clinical trial evaluating the use of Hansen Medical's Artisan family of control catheters with its Sensei X Robotic Catheter System for treatment of atrial fibrillation.
eCardio appoints John H Untereker as new president
On 1 August, eCardio Diagnostics announced the promotion of John H Untereker to president, effective immediately.
Physicians should counsel patients about sex life after cardiac event
Healthcare professionals are urged to counsel heart and stroke patients on how to resume a healthy sex life, according to a joint statement published in the American Heart Association journal Circulation and the European Heart Journal.
New findings for acute heart failure treatment with Trevena’s biased ligands
Trevena has announced the publication of new findings related to the mechanism of action of its Angiotensin II Type 1 Receptor (AT1R) biased ligands. The publication describes work led by R John Solaro, head of the Department of Physiology and Biophysics at the University of Illinois, Chicago, USA, performed in collaboration with Trevena scientists.
FDA clears Vdrive Robotic Navigation System with V-Sono Intracardiac Echocardiography catheter...
The Vdrive with V-Sono system (Stereotaxis) is indicated for the remote control of compatible intracardiac echocardiography or ultrasound catheters inserted into the right atrium for cardiac ablation procedures.
Quality of life overestimated or underestimated in 55% of paroxysmal atrial...
A substudy from the ANTIPAF trial has found that quality of life was overrated or underrated by physicians in 55% of patients with paroxysmal atrial fibrillation. Depression was the major contributor to a higher likelihood of overrating.
Coil embolisation now used to treat ventricular tachycardias
Physicians in the USA are using, for the first time, coil embolisation to treat severe ventricular tachycardias. The intervention is normally employed for treating brain aneurysms. Cardiac Rhythm News spoke to Kalyanam Shivkumar, co-author of the report in more detail about the technique, its benefits and most suitable patients for this procedure.
100th SynCardia Total Artificial Heart implanted in 2013
On 25 July, SynCardia Systems announced that 100 SynCardia temporary Total Artificial Hearts have been implanted by SynCardia certified centres in 2013.
FDA clears Rhythmia Mapping System
The Rhythmia Mapping System (Boston Scientific) is a next-generation 3D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology procedures to diagnose or treat cardiac arrhythmias.
Remote monitoring of ICD patients: What is the patients’ opinion?
Werner Jung (Villingen-Schwenningen, Germany) revises patients' opinions regarding remote monitoring of implantable cardiac defibrillators (ICDs). He spoke on the subject at the European Heart Rhythm Association (EHRA) Europace meeting (23-26 June, Athens, Greece).
ESC and AEPC release consensus statement on paediatric arrhythmias
The consensus on paediatric arrhythmias is the first European statement concerning the diagnosis and management of paediatric arrhythmias. It is also the first joint document between European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC) and the Association for European Paediatric and Congenital Cardiology AEPC.
British Heart Foundation awards Zoll a tender for automated external defibrillators...
On 17 July, Zoll Medical announced that it has been selected as a nominated supplier by the British Heart Foundation (BHF) for the Zoll AED Plus through 2016.
mHealth report forecasts up to US$36 billion savings from remote patient...
Juniper Research has announced that its latest report on the mHealth (mobile health) market forecasts cumulative cost savings from remote patient monitoring of up to US$36 billion globally over the next five years.
Dabigatran shows sustained benefit in difficult to treat patients with atrial...
Results from a new sub-analysis of the RE-LY trial support consistent benefit of dabigatran (Pradaxa, Boehringer Ingelheim) over warfarin in difficult-to-treat patients with non-valvular atrial fibrillation and previous symptomatic heart failure.
Is atrial fibrillation a genetic condition?
Despite the widespread nature of atrial fibrillation, our understanding of the disease remains limited according to Patrick T Ellinor, director Cardiac Step Down Unit, Massachusetts General Hospital Corrigan Minehan Heart Centre (Boston, USA) and associate professor, Harvard Medical School. He spoke to Cardiac Rhythm News about current research on genetics of atrial fibrillation.
David Francischelli is the new vice president of Research and Development...
AtriCure has announced that David Francischelli has been appointed as vice president of Research and Development, effective 6 August 2013.
FDA clears fully resorbable AIGISRx R Antibacterial Envelope
AIGISRx R is a fully bioresorbable, antibacterial mesh envelope, intended to hold cardiovascular implantable electronic devices securely in place in order to provide a stable environment when implanted in the body.
The impact of radiofrequency ablation for atrial fibrillation on the gastrointestinal...
There have been a number of studies conducted to assess the efficacy and adverse effects of radiofrequency ablation of atrial fibrillation, but very little data is available on the effect of radiofrequency ablation on the upper gastrointestinal system. Dhanunjaya Lakkireddy, Kansas City, USA, reviews this subject based on the results of a new systematic approach the AF GUT study.
A good night’s sleep increases the cardiovascular benefits of a healthy...
A good night's sleep can increase the benefit of exercise, healthy diet, moderate alcohol consumption and non-smoking in their protection against cardiovascular disease, according to results of a large population follow-up study, the MORGEN study, published on 3 July in the European Journal of Preventive Cardiology.
First pulmonary vein isolation procedure combined with renal denervation for atrial...
According to a Mass General press release, this is the first hospital in New England -and the second in the USA-to pair renal artery sympathetic denervation with pulmonary vein isolation for patients with atrial fibrillation and hypertension.
Complete pulmonary vein isolation more effective in atrial fibrillation treatment
Complete linear lesions when using catether ablation to isolate pulmonary veins have been shown to be more effective than incomplete lesions in preventing recurrence of atrial fibrillation, according to results from the GAP-AF study presented at a late-breaking trial session of the European Heart Rhythm Association (EHRA) Europace meeting (23-26 June, Athens, Greece).
Multicentre European study set to evaluate HeartLight system for persistent atrial...
A study is to be the first to prospectively assess the outcomes of pulmonary vein isolation with balloon ablation catheters in patients with persistent atrial fibrillation, according to a company release.
Lower electrode-related complication rate for S-ICD system vs transvenous ICD leads
Outcomes of an analysis, presented at European Heart Rhythm Association (EHRA) Europace 2013, followed recent NICE guidance supporting safety and effectiveness of subcutaneous implantable defibrillators.
St Jude medical launches wireless MRI-conditional pacemaker in Japan
The Accent MRI Pacing System was said, in a company release, allows the use of full-body scans using high-power imaging technology in patients with the pacemaker.
Boston Scientific to acquire Bard EP
Boston Scientific has announced that it has entered into a definitive agreement to acquire Bard EP, the electrophysiology business of CR Bard, for US$275 million in cash. The company expects to close the transaction in the second half of 2013, subject to customary closing conditions.
Ablation superior to medical therapy for patients with persistent atrial fibrillation
According to results from the SARA study, presented at a late-breaking trial session of the European Heart Rhythm Association (EHRA) Europace meeting (23-26 June, Athens, Greece), catheter ablation therapy has been shown to be superior to medical therapy for maintenance of sinus rhythm in patients with persistent atrial fibrillation.
AliveCor’s mobile ECG now available in UK and Ireland
AliveCor has announced that its Heart Monitor for iPhone 4, 4S and 5 is now available for purchase in the UK and Ireland. The device will be on display at the European Heart Rhythm Association Europace 2013 meeting.
St Jude Medical receives CE mark approval of multipoint pacing CRT-D
St Jude Medical has announced that is has received CE mark approval of its next-generation quadripolar device, the Quadra Assura MP cardiac resynchronisation therapy defibrillator (CRT-D).
Sorin announces results of the DREAM clinical study
In this study, the sleep apnoea monitoring (SAM) algorithm of Sorin Reply 200 pacemakers was validated against polysomnography, the gold standard method used to measure the severity of sleep apnoea.
Researchers find a new possible cause of sudden infant death
Researchers in USA and Australia have found a new potentially treatable cause of sudden infant death, consisting of idiopathic ventricular fibrillation preceded by ST segment changes and QRS widening.
Andreas Goette
Andreas Goette is the chair of the European Heart Rhythm Association (EHRA) Europace Scientific Programme Committee. He spoke to Cardiac Rhythm News about his research in molecular electrophysiology, a subject which was absent 15 years ago. He also gave his views on antiarrhytmic drugs and catheter ablation technology, and spoke about the highlights of EHRA Europace 2013.
New risk assessment tool to predict stroke in patients with atrial...
Model will give physicians more reliable guidance in making therapeutic decisions for stroke prevention.
Wearable defibrillator aids decision making on ICD need
A management programme that incorporates a wearable cardioverter defibrillator can be used to bridge a decision for appropriate implantable cardioverter defibrillator (ICD) therapy in patients with acquired, inherited and congenital heart disease, according to 18-month results from the WEARIT-II registry presented at Heart Rhythm 2013
Biotronik launches Ilesto 7 Series in Europe
Biotronik has announced the European market launch of its Ilesto 7 Series. This is the world's first DF4 implantable cardiac defibrillator (ICD) and cardiac resynchronisation therapy (CRT) series approved for MRI.
Promising mid-term results from thoracoscopic ablation of persistent atrial fibrillation
At three-year follow-up, 80% of patients with persistent atrial fibrillation showed freedom from atrial fibrillation and were free from anti-arrhythmic drugs after thoracoscopic ablation with the Bipolar RF Ablation System (AtriCure).
Elderly people benefit from using implantable defibrillators
Elderly people may benefit from implantable cardioverter defibrillators as much as younger people, according to new research published in Circulation.
RELY-ABLE results support long-term safety of dabigatran in non-valvular atrial fibrillation...
Results from the RELY-ABLE trial, a long-term extension of the pivotal RE-LY study of dabigatran (Pradaxa / Boehringer Ingelheim) in patients with non-valvular atrial fibrillation, have provided additional information showing long-term safety of dabigatran.
Sudden cardiac death in young athletes under study
The Christ Hospital Health Network, the University of Mississippi Medical Center and Cincinnati Children's Hospital Medical Center have partnered with USRowing and Toshiba to conduct The Athlete Heart Research Study, which aims to determine if sudden cardiac death can be prevented with a heart screening.
Long-distance cross-country skiers at increased risk of cardiac arrhythmias
Cross-country skiers who take part in one of the world's most challenging ski races, the 90km Vasaloppet in Sweden, are at increased risk of developing cardiac arrhythmias according to a study of nearly 53,000 race participants published online in the European Heart Journal.
Sleep apnoea increases risk of sudden cardiac death
A moderate case of obstructive sleep apnoea can significantly increase a person's risk for sudden cardiac death, according to the largest study of its kind published online in the Journal of the American College of Cardiology.
FDA approves next generation Ellipse and Assura ICD’s and CRT-Ds
St Jude Medical's next-generation devices feature additional safety features to address common lead complications.
American College of Cardiology and American Heart association update guidelines for...
The updated guideline emphasises on patient-centric outcomes such as quality of life, shared decision making, care coordination, transitions, and palliative care.
Inappropriate shocks may be reduced with dual-chamber ICDs
Results from the OPTION study have shown that patients with dual-chamber implantable cardioverter defibrillators (ICD) experienced a significantly lower incidence of inappropriate shocks compared with patients with standard single-chamber devices (4.3% vs.10.3%, p=0.015).
Effectiveness of left atrial appendage exclusion, ligation and occlusion in reducing...
Andre d'Avila and Arash Aryana write on three different left atrial appendage strategies aimed to reduce stroke risk in atrial fibrillation patients. d'Avila presented this analysis at Heart Rhythm (Denver, USA, 8-5 May 2013).
John D Day
John D Day is the medical director at Intermountain Heart Rhythm Specialists, Utah, USA. He spoke to Cardiac Rhythm News about his career achievements, the atrial fibrillation ablation programme at the Intermountain Heart Institute, his research on atrial fibrillation and Alzheimer's disease.
Shock-related anxiety from heart devices is associated with sexual dysfunction in...
The multicentre study, published in the June edition of HeartRhythm, is the first to discover that shock-related anxiety is associated with sexual dysfunction in young adults and calls on healthcare providers to address these issues to improve the quality of life for patients.
First-in-man study shows promise with leadless pacing
At the Heart Rhythm Society (HRS) in Denver, USA, (8-11 May), Vivek Reddy, Mount Sinai School of Medicine, New York, USA, told delegates that leadless right ventricular cardiac pacing is feasible.
FDA conditionally approves new study design for the Artisan family of...
The US Food and Drug Administration (FDA) has given conditional approval to Hansen Medical to change the company's study design of its ARTISAN-AF trial.
Death highest in heart failure patients admitted in January, on Friday,...
Mortality and length of stay are highest in heart failure patients admitted in January, on Friday, and overnight, according to research presented at the Heart Failure Congress 2013 in Lisbon, Portugal (25-28 May).
RELAX-AHF and VIVIDD trial results presented at Heart Failure Congress
Serelaxin may be more effective for relieving dyspnoea in heart failure with preserved ejection fraction (HFpEF) than reduced (HFrEF) during the first 24 hours, according to results from RELAX-AHF presented at a late-breaking trial session at the Heart Failure Congress (Lisbon, Portugal, 25-28 May).
IMPACT study of Biotronik Home Monitoring completes enrolment
The IMPACT study aims to investigate whether the use of Biotronik Home Monitoring in conjunction with anticoagulation can reduce the risks of stroke, systemic embolism and major bleeding in cardiac device patients.
Continued warfarin better than heparin bridging during device implantation
A randomised trial (BRUISE CONTROL) has shown that performing device implantation surgery without interruption of warfarin in patients with an annual risk of stroke equal to or over 5% was associated with a significant reduction in device pocket haematoma. Data was presented at the Heart Rhythm Society (HRS) meeting in Denver, USA.
What’s hot at EHRA Europace 2013
The European Society of Cardiology (ESC) has announced that this years' European Heart Rhythm Association (EHRA) Europace meeting will include, for the first time, late-breaking clinical trial sessions.
Biventricular pacing yields superior results than conventional right-ventricular pacing in BLOCK...
Data from the BLOCK HF trial demonstrated that simultaneously pacing the lower chambers of the heart, or biventricular (BiV) pacing with a cardiac resynchronization therapy (CRT) device, significantly improves heart failure symptoms and quality of life in patients with atrioventricular block and left ventricular systolic dysfunction.
UK’s NICE supports improved commissioning for anticoagulation therapy for adults
On 14 May, UK's National Institute for Health and Care Excellence (NICE) issued updated support for commissioners to help them work with clinicians and managers to commission high-quality, evidence-based anticoagulation therapy for adults across England.
Targeted ablation without pulmonary vein isolation shows 82% single procedure success...
A late breaking trial presented at Heart Rhythm 2013 (8-11 May, Denver, USA) has demonstrated that targeted ablation of stable rotors and focal sources can eliminate paroxysmal atrial fibrillation without the need to isolate the pulmonary veins.
Biocontrol Medical launches new website for patient and clinician education on...
BioControl Medical has introduced a new corporate website that offers patients, family and clinicians an educational resource on its CardioFit therapy for heart failure, as well as the INOVATE-HF clinical study of the device.
Use of antibacterial envelope associated with very low 90-day cardiac device...
Results from the CITADEL/CENTURION study have shown 95% fewer major cardiovascular implantable electronic device (CIED) infections in patients undergoing CIED replacement procedures using an antibacterial envelope (AIGISRx, Tyrx).
Consensus statement on inherited primary arrhythmias syndromes released
Three scientific societies present the first comprehensive recommendations on patients with inherited arrhythmias at Heart Rhythm 2013
Rhythmia Mapping System gets CE mark
The Rhythmia Mapping System (Boston Scientific) is a next-generation 3D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology procedures to treat cardiac arrhythmias including: atrial flutter, atrial fibrillation and ventricular tachycardia.
Contact force highlighted at HRS 2013
Endosense presented new study data validating the importance of several proprietary contact force parameters in cardiac ablation procedures, including its most recently developed Lesion Index (LSI), at Heart Rhythm 2013.
FDA approves Smartview remote monitoring system
The Smartview remote monitoring solution (Sorin) in conjunction with the Sorin Paradym RF device allows valuable cardiac data and alert messages from the device while the patient is at home.
GE Healthcare showcases Innova EPVision 2.0 and new dose reduction technologies...
GE Healthcare introduced, at HRS, the Innova EPVision 2.0 an application that combines information from EP recording and live fluoroscopy, and Dose Blueprint.
Independent analysis shows positive results for Durata and Riata leads at...
At HRS, John Cairns, on behalf of the Population Health Research Institute (PHRI), presented five-year follow-up results from an independent analysis of over 10,000 Durata and Riata ST Optim implantable cardioverter defibrillator leads.
Next generation Ellipse and Assura ICDs and CRT-Ds get CE mark...
On 8 May, St Jude Medical announced the CE mark approval of its next-generation Ellipse and Assura portfolio of implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds).
Boston Scientific completes first-in-human clinical trial of the IntellaTip MiFi XP...
A company release states that this new line of ablation catheters is designed to provide physicians with high resolution, precise, multidimensional information through sophisticated micro-sensors at the tip of the catheter.
ICDs programmed with a long-detection interval reduces anti-tachycardia pacing, ICD shocks...
A study published in JAMA shows that programming an implantable cardioverter-defibrillator (ICD) with a long-detection interval compared with a standard-detection interval resulted in a reduction in anti-tachycardia pacing episodes, ICD shocks delivered, and inappropriate shocks.
Laser lead extraction technology to be showcased at Heart Rhythm 2013
Spectranetics has announced that the company will have a major presence to showcase their proprietary laser lead extraction technology and training at the Heart Rhythm 2013 congress in Denver, USA.
Pediatric and Congenital Electrophysiology Society recommends higher standards for the advancement...
The Training and Credentialing Committee of the Pediatric and Congenital Electrophysiology Society (PACES) has launched a comprehensive review of advanced electrophysiology fellowships in an effort to ensure the highest possible standards for training programmes and practitioners in the field.
FDA approves Ilesto 7 ICD/CRT-D series
According to a company release, these devices are smaller, thinner and lighter without compromising clinical capabilities.
FDA approves Viva CRT-D and Evera ICDs
On 6 May, Medtronic announced the FDA approval and US launch of its newest cardiac devices: the Viva portfolio of cardiac resynchronisation therapy with defibrillation (CRT-D) devices, and the Evera portfolio of implantable cardioverter-defibrillators (ICD).
First patient enrolled in next-generation Quadra Study
The MultiPoint Pacing study (St Jude Medical) is designed to show improved effectiveness for patients when pacing multiple locations of the heart.
Renal denervation now under consideration for arrhythmias
Studies are exploring renal denervation as an adjunctive treatment in other diseases characterised by sympathetic overactivity such as ventricular arrhythmias, atrial fibrillation, metabolic syndrome and obstructive sleep apnoea. Cardiac Rhythm News spoke to leading physicians who are investigating this technology for the management of atrial fibrillation.
FDA approves Kcentra for urgent warfarin reversal in patients with acute...
Kcentra is the first FDA-approved 4-factor prothrombin complex concentrate for warfarin reversal in the USA.
Peng-Sheng Chen: new editor-in-chief of HeartRhythm
Chen set to begin term as second-ever editor-in-chief of the official journal of the Heart Rhythm Society effective 1 January 2014
Allure Quadra quadripolar CRT pacemaker gets CE mark
Allure Quadra (St Jude Medical) is the first-to-market quadripolar cardiac resincronisation system that allows for increased implant efficiencies that result in fewer surgical revisions, according to a company release.
CVRx launches HOPE4HF clinical trial evaluating Barostim neo in heart failure...
The Barostim neo device is an alternative treatment to drug therapy designed to trigger the body's own natural blood flow regulation system to treat heart failure.
Hansen Medical to highlight Sensei Robotic Catheter System and new Artisan...
On 29 April, Hansen Medical announced that it will showcase its Sensei X Robotic Catheter System with the new Artisan Extend Catheter at the Heart Rhythm Society's (HRS) 34th Annual Scientific Sessions (8-11 May, Denver, USA).
ESC and EHRA release guide on new oral anticoagulants use
According to the European Society of Cardiology (ESC), the guide was designed responding to the need to summarise existing information on different drugs, to answer clinical questions that fall outside what drug companies can legally answer, and to make distinctions between the different drugs.
Atrial fibrillation may be reduced in sleep apnoea patients using airway...
Continuous positive airway pressure may help to reduce atrial fibrillation recurrence in patients with obstructive sleep apnoea undergoing pulmonary vein isolation, a study published ahead of print in the Journal of the American College of Cardiology (JACC) has found.
Remote monitoring and worldwide adoption: Is it already changing clinical practice?
Thomas Vogtmann reviews expert consensus and guidelines on the adoption of remote monitoring, and discusses how clinical practice might change for the better as a result of its increased usage. He gave this talk at the XV International Symposium on Progress in Clinical Pacing in Rome, Italy
Leading physicians launch avert-AF initiative to develop drug treatment for atrial...
Cardiovascular scientists from the University of Michigan, USA, and St George's University of London, UK, announced the launch of avert-AF, a new initiative aimed at developing drug treatments for the prevention of the more advanced forms of atrial fibrillation.
Shalom Jacobovitz Named CEO of American College of Cardiology
On 22 April, the board of trustees of the American College of Cardiology (ACC) announced that Shalom "Shal" Jacobovitz has been selected as the college's chief executive officer.
Cell therapy and high-dose ultrasound treatment may help to improve cardiac...
A new treatment using shock wave (procedure using high-dose ultrasound) and mononuclear cells was associated with significant, albeit modest improvement in left ventricular ejection fraction (LVEF) after four months in patients with chronic postinfarction heart failure.
Electrocardiographic abnormality associated with increased risk of atrial fibrillation, congestive heart...
Left anterior fascicular block may be a clinically relevant marker of an individual's propensity to develop atrial fibrillation or congestive heart failure, a new study has found.
Boston Scientific begins SAMURAI trial to evaluate new pacing system in...
The first patient has been implanted with the next generation ImageReady MR Conditional pacing system (Boston Scientific) in the SAMURAI clinical trial.
HeartLight system featured at German Cardiac Society Meeting
CardioFocus has announced that presentations of the HeartLight system and its role in the treatment of advanced atrial fibrillation were featured at the 79th Annual Meeting of the German Cardiac Society in Mannheim, Germany.
First implantation of AIGISRx R Fully Resorbable Antibacterial Envelope performed in...
On 15 April, TYRX announced that the first ever implantation of its new AIGISRx R Fully Resorbable Antibacterial Envelope took place at the Quebec Heart and Lung Institute in Quebec City, Canada by François Philippon.
Spectranetics’ Lead Extraction Simulator goes mobile in the USA
Spectranetics has announced that the lead extraction simulator, a virtual, hands-on environment to train physicians in the removal of pacemaker and defibrillators leads, will be taken directly to the physicians and the hospitals where they practice with a fully mobile version.
Inliven CRT-P device gets CE mark to treat heart failure
Inliven (Boston Scientific) is the first cardiac resynchronisation therapy pacemaker (CRT-P) using patient's respiration signals to provide physiological pacing and comorbidity management.
SynCardia certified centres to present Total Artificial Heart abstracts at International...
The topics include home discharge using the Freedom Portable Driver and the use of the Total Artificial Heart for adolescents, congenital patients and destination therapy.
Medtronic launches CardioGuide Implant System to guide cardiac resynchronisation therapy implants
The system helps physicians determine the most appropriate location for left-ventricular lead placement by generating 3-D images of the cardiac veins.
EFFICAS I study yields new contact force guidelines in catheter ablation...
Results from the EFFICAS I, a prospective multicentre study sponsored by Endosense, have led to the development of guidelines for target and minimum contact force, as well as minimum force time integral, during the catheter ablation treatment of paroxysmal atrial fibrillation.
Biotronik’s Iforia ICD/CRT-D series get the CE mark
According to a company release, Iforia is the world's first DF4 ICD/CRT-D series approved for magnetic resonance imaging.
FDA issues proposal to improve the quality of automated external defibrillators
The FDA announced that if the proposed order is finalised it will require manufacturers of these life-saving devices to submit pre-market approval applications.
Stereotaxis receives Japanese regulatory approval of Niobe technology for cardiac ablations
Approval by the Japanese Pharmaceuticals and Medical Devices Agency follows a successful three-year clinical trial of the Niobe remote magnetic technology for cardiac ablations at the Tokyo Women's Medical University.
PREVAIL shows Watchman device is safe; efficacy is still under discussion
Preliminary results of the PREVAIL trial have demonstrated the Watchman device (Boston Scientific / Atritech) is a safe alternative to oral anticoagulation therapy for stroke prevention in non-valvular atrial fibrillation patients. However, there are now questions towards Watchman's efficacy.
Latest National ICD Registry captures data of about “90% of all...
Cardiac Rhythm News speaks to Mark S Kremers, chairman, ICD Registry Steering Committee, on the results from the latest US ICD registry led by the Heart Rhythm Society and the American College of Cardiology Foundation. In this registry, Kremers said: "about 90% of all ICDs implanted in the USA are tracked".
Ventricular arrhythmia episodes may decrease after renal denervation in resistant hypertensive...
A small study presented at ACC 2013 has shown that renal denervation, besides reducing resistant hypertension, produces a favourable effect on atrial and ventricular arrhythmias.
FDA gives conditional approval to Sorin to conduct RESPOND CRT clinical...
The trial will study the safety and effectiveness of the SonR cardiac resynchronisation therapy (CRT) optimisation system in patients with advanced heart failure.
Leadless technology: the next frontier of cardiac pacing
There are many concerns on leadless technology. However, with the increase recognition of lead related problems and advantages of endocardial pacing, leadless technology will be the next frontier of cardiac pacing. Chu-Pak Lau, Hong Kong, writes for Cardiac Rhythm News.
Newest version of the US National ICD Registry available now
The updated registry, developed through a partnership of the Heart Rhythm Society and the American College of Cardiology Foundation, captures lead data and paediatric procedures for the first time.
American College of Cardiology and Boehringer Ingelheim collaborate to advance physician...
In parallel with the American College of Cardiology (ACC) Scientific Sessions in San Francisco, USA, initial results have been released from a physician educational programme being undertaken to rectify misunderstandings on stroke prevention in atrial fibrillation in China.
Azithromycin could potentially provoke fatal heart rhythms, FDA warns
The FDA has issued a Drug Safety Communication as a result of its review of a study by medical researchers as well as another study by Pfizer that assessed the potential for azithromycin to cause abnormal changes in the electrical activity of the heart.
Zio Patch shows improved paroxysmal atrial fibrillation detection compared with 24-hour...
Zio Patch, anambulatory cardiac monitoring system (iRhythm), has shown benefit of long-term monitoring or paroxysmal atrial fibrillation patients and significant impact on treatment strategy.
AtriCure appoints Robert S White to its board of directors
Robert S White is president and CEO of TYRX, the company behind the AIGISRx Antibacterial Envelope, which is designed to reduce surgical site infections associated with cardiac implantable electronic devices.
Weight loss may increase risk of cardiac events in patients implanted...
Even minor weight loss is associated with worse health outcomes among patients implanted with cardiac resynchronisation therapy with defibrillator (CRT-D), according to research presented by Valentina Kutyifa, Rochester, USA, at ACC 2013.
First patient implanted with the Amplatzer Cardiac Plug device in ACP...
The ACP (Amplatzer Cardiac Plug) clinical trial is designed to determine if the Amplatzer device (St Jude Medical) is safe and effective in preventing thrombus from migrating out of the left atrial appendage in patients with non-valvular atrial fibrillation who have a high risk for stroke.
PREVAIL preliminary results of Watchman device to be revealed at ACC...
Boston Scientific will present, for the first time, preliminary results of all three co-primary endpoints of the Watchman device at the American College of Cardiology 2013 Annual Scientific Sessions.
Syncope events in bifascicular block patients can be reduced with permanent...
Massimo Santini, Rome, Italy, and others have recently published results from the PRESS study. They found that by using a dual chamber pacemaker programmed to 60ppm lower rate the number of syncope events can be reduced in bifascicular block patients.
Myocardial fibrosis may be an independent predictor of malignant ventricular arrhythmia
Researchers in the United Kingdom have found in a first-of-its-kind study that fibrosis detected by late gadolinium enhancement cardiac MRI may be an independent predictor of hard cardiovascular events in patients with ventricular arrhythmias.
The American College of Cardiology and Elsevier launch a journal dedicated...
JACC: Heart Failure is a new journal dedicated to meet the demands and challenges of treating what is now the most rapidly increasing category of cardiac disease. The first publication is now available.
Endosense closes an additional US$4.3 million to finance ablation technology
The added finances will further support Endosense's programme to get access to the US market with its TactiCath Quartz contact-force sensing ablation catheter and to reinforce sales in Europe.
Insomnia is linked to increased risk of heart failure
People who suffer from insomnia appear to have an increased risk of developing heart failure, according to a study published online in the European Heart Journal.
Attain Performa quadripolar leads are CE-marked
The Attain Performa leads (Medtronic) are designed with shorter spacing between the two centre electrodes to increase phrenic nerve thresholds and reduce phrenic nerve stimulation, a company release states.
HeartLight Endoscopic Ablation System to be highlighted at Frankfurt Atrial Fibrillation...
The Annual Frankfurt Atrial Fibrillation Academy Symposium will highlight the latest innovations and research in atrial fibrillation therapy.
Appropriate use criteria for ICDs and CRT released
The document, developed by the American College of Cardiology, the Heart Rhythm Society and other key specialty societies, examines potential clinical scenarios to support physician decision making.
Isis Pharmaceuticals initiates phase 2 study of ISIS-CRP(Rx) in atrial fibrillation...
ISIS-CRP(Rx) is being evaluated in patients with atrial fibrillation to measure the effect of lowering C-reative protein on the frequency and duration of the episodes.
Newer approaches in ablation for ventricular fibrillation
"Ventricular fibrillation was generally viewed as a non-ablatable rhythm disturbance. However, several advances over the last decade have enabled electrophysiologists to successfully address this arrhythmia with ablation techniques," writes Samuel J Asirvatham, Mayo Clinic, Rochester, USA.
UK’s NICE publishes final guidance recommending apixaban to prevent stroke in...
On 27 February, the UK's National Institute for Health and Clinical Excellence (NICE) released final guidance recommending apixaban (Eliquis, Bristol-Myers Squibb and Pfizer) as an option for the prevention of stroke and systemic embolism in some people with non-valvular atrial fibrillation.
Sorin Group announces the acquisition of Alcard
Alcard is a Brazilian leading manufacturer of medical devices for cardiac surgery.
FDA approves first single-lead ICD with atrial sensing
The Lumax 740 DX System from Biotronik expands the diagnostic capabilities of a standard single-chamber ICD with a single lead, in addition to featuring sophisticated sensors that allow for atrial monitoring and enhanced arrhythmia diagnosis.
Massachusetts General Hospital launches the Institute for Heart, Vascular and Stroke...
According to a Massachussetts General Hospital press release this is one of the only institutes in the world to integrate cardiovascular and cerebrovascular care.
“Not all ICD shocks are harmful”
A new sub-analysis of the MADIT-CRT multicentre trial has shown that implantable cardioverter defibrillator (ICD) shocks at the time of defibrillation threshold testing in patients with mild symptoms of heart failure are not associated with an increased risk of heart failure or death or future episodes of ventricular tachycardia and ventricular fibrillation.
Magnetic resonance imaging at 1.5 Tesla is safe in patients implanted...
According to study results of the Advisa MRI System clinical study, the system, recently approved by the FDA, allows magnetic resonance imaging access without compromising positioning restrictions, including the cardiac region.
European Heart Rhythm Association produces a practical guide to radiation protection
Despite the advent of non-fluoroscopic technology, fluoroscopy remains an imaging cornerstone, both in interventional electrophysiology and in device implantation. Moreover, many patients receive additional X-ray imaging, such as cardiac CT and others. Hein Heidbuchel outlines a new European Heart Rhythm Association (EHRA) guide to radiation protection.
CardioNet and AirStrip partner to expand mobile patient cardiac monitoring
The AirStrip mobility solution will deliver live patient data from CardioNet's Mobile Cardiac Outpatient Telemetry (MCOT) directly to clinicians' mobile devices, including tablets and phones.
Americans ignore warning signs of atrial fibrillation, a new survey from...
The Heart Rhythm Society calls on all Americans to know risks and warning signs of the most common arrhythmia during February the Heart Health Month.
Ilesto 7 ICD/CRT-D Series with ProMRI technology gains CE mark approval
The Ilesto 7 series ( Biotronik) includes one of the world's smallest ICDs and features ProMRI technology, which enables access to potentially life-saving MR scans.
Mortara Instrument releases ELI 280 resting electrocardiograph
The ELI 280 is a 12-lead resting electrocardiograph featuring a tablet-style touch screen user interface .
FDA approves Advisa MRI Pacemaker System
The Advisa MRI system is Medtronic's second-generation MR-Conditional pacemaker and, according to a company release, is the first system to combine the most advanced pacing technology with proven magnetic resonance imaging (MRI) access.
The Scottish Medicines Consortium accepts apixaban for prevention of Stroke in...
The Scottish Medicines Consortium has accepted the novel oral anticoagulant apixaban (Eliquis, Bristol-Myers Squibb / Pfizer) for use within NHS Scotland for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors.
Statin therapy is borderline significantly associated with depression and physical functioning...
Researchers in The Netherlands have found that there is a borderline significant association between statin therapy and depression and statin therapy and physical functioning in patients with an implantable cardioverter defibrillator.
Long-term results of PROTECT AF confirm left atrial appendage closure is...
Vivek Y Reddy, Mount Sinai School of Medicine, New York, USA, and others recently reported the updated results ahead of print in Circulation.
Fully Automatic AED Plus granted FDA 510(k) clearance
The new fully automatic unit provides all the same Full-Rescue features and benefits as the semiautomatic version of the AED Plus except that a shock is delivered automatically if one is advised.
An approach to patient education and raising greater awareness of heart...
The Heart Rhythm Society has several campaigns to raise awareness of heart rhythm disorders, such as "Arrest the Risk Awareness" that aims to raise awareness of sudden cardiac arrest among black and Hispanic communities. Anne Gillis reviews the importance of patient education.
Heart Rhythm Society and Association of Black Cardiologists launch 10-City Arrest...
The Heart Rhythm Society and the Association of Black Cardiologists are working in conjunction to help reduce the incidence of sudden cardiac arrest among African Americans through a new campaign titled 10-City Arrest the Risk.
Rivaroxaban to be studied with Factor Xa inhibitor antidote
Janssen Pharmaceuticals in conjunction with Portola Pharmaceuticals and Bayer HealthCare will evaluate several dosage strengths of PRT4445 and its ability to reverse the anticoagulant activity of rivaroxaban in emergency situations.
Minnesota Timberwolves star Ricky Rubio teams up with Medtronic Foundation to...
Now with the help of Rubio, the HeartRescue Project is unveiling the latest life-saving innovation, an interactive online experience called the "Save-A-Life Simulator".
Biotronik Home Monitoring is efficient and effective, MoniC study shows
The MoniC study, conducted at the Campus Charite Mitte, Berlin, addressed the feasibility, safety, workload and clinical usefulness of a centralised Biotronik Home Monitoring model.
GE Healthcare introduces new cardiac MRI analysis software
GE Healthcare has announced two new software packages for advanced analysis of cardiovascular magnetic resonance (MR) images: CardiacVX and MR VesselIQ Xpress.
Evera implantable cardioverter-defibrillators get the CE mark
The new Evera ICD portfolio (Medtronic) features increased longevity and advanced shock reduction technology.
First US case performed with Endosense’s Tacticath Quartz contact-force sensing ablation...
The first US case was performed on 31 January 2013 as part of an amended protocol of the company's TOCCASTAR investigational device exemption clinical study of the TactiCath called the TOCCASTAR Supplemental Clinical Study.
Marriage reduces the risk of heart attack for men and women
A large population-based study from Finland, published in the European Journal of Preventive Cardiology, has shown that being unmarried increases the risk of fatal and non-fatal heart attack in both men and women whatever their age.
New AIGISRx R Fully Resorbable Antibacterial Envelope receives approval from Health...
The new envelope includes the advantages of the original AIGISRx, such as stability and implantable electronic devices' infection reduction, but now with the added benefit of being fully resorbable.
Yoga helps atrial fibrillation patients
A first-of-its-kind study by Dhanunjaya Lakkireddy, Kansas, USA, and others has found that rigorous practice of yoga can help reduce episodes of irregular heartbeat and improve the symptoms of anxiety and depression often associated with atrial fibrillation.
Update on GENETIC-AF trial for Gencaro in Atrial Fibrillation
GENETIC-AF is projected to be a 620-patient, phase 3 study comparing Gencaro (ARCA biopharma) to metoprolol CR/XL for prevention of atrial fibrillation in patients with heart failure and left ventricular dysfunction.
The use of dabigatran in clinical practice
Dabigatran (Pradaxa, Boehringer Ingelheim) was the first of three novel anticoagulants, the others being rivaroxaban (Xarelto, Bayer) and apixaban (Eliquis, Bristol Myers Squibb, Pfizer), to be approved for stroke prevention in patients with non-valvular atrial fibrillation. Giuseppe Di Pasquale reviews the use of the drug in clinical practice.
Defibrillator manufacturer signs pledge to make patient data available to reduce...
ZOLL Medical has announced that ZOLL is the first major defibrillator manufacturer to publicly pledge the sharing of patient data to reduce preventable deaths.
Faster lower pacing rate after atrioventricular node ablation may help to...
Faster lower pacing rate immediately after atrioventricular node ablation with a gradual decrease helps to improve clinical outcome and may reduce the risk of sudden death in patients with atrial fibrillation, Ru-Xing Wang and other reported in Heart Rhythm ahead of print.
Biotronik to start MATRIX study to explore improved detection and management...
MATRIX is a prospective open study that will include 2,000 patients in 100 sites throughout Europe, Latin America, Canada, Israel and Japan for up to four years.
FDA clears Lifeline PRO and Lifeline ECG automated external defibrillators
These external defibrillators feature a revolutionary color LCD video display and 3-lead ECG monitoring capability.
Gregory YH Lip
Gregory YH Lip, Birmingham, UK, talked to Cardiac Rhythm News about the highlights of his career, including his mother as his biggest influence, and the recent update to the European Society of Cardiology (ESC) guidelines for the management of atrial fibrillation.
Are patients with atrial fibrillation at increased risk of developing dementia?
Pasquale Santangeli, Texas Cardiac Arrhythmia Institute, St David's Medical Centre, Austin, USA, spoke to Cardiac Rhythm News about the link between atrial fibrillation and dementia.
NICE recommends apixaban for some people with non-valvular atrial fibrillation
On 23 January, the UK's National Institute for Health and Clinical Excellence (NICE) issued a final draft guidance recommending apixaban (Eliquis, Bristol-Myers Squibb and Pfizer), in accordance with its licensed indications, for the prevention of stroke and systemic embolism in some people with non-valvular atrial fibrillation.
Clinical trial finds CorMatrix ECM technology does not increase incidence of...
Study data suggest it is safe to use CorMatrix ECM technology to close the pericardium of first-time coronary artery bypass graft patients.
St Jude Medical receives the CE mark for the Amplatzer Amulet...
The Amulet device is used to close the left atrial appendage (LAA) in patients diagnosed with non-valvular atrial fibrillation.
FDA clears EPi-Sense cardiac ablation device with embedded sensors
This next generation of technology includes sensors along the ablation device which provide real-time feedback to physicians conducting cardiac ablation procedures.
CardioFocus HeartLight US pivotal trial on track for full enrolment in...
The HeartLight pivotal trial is randomising the HeartLight system against the ThermoCool Catheter, and will randomise an estimated 350 patients in the multicentre US trial.
Lifeline Auto automatic external defibrillator cleared for sale in the USA
The fully automatic external defibrillator system, which has been developed by Defibtech, was designed to provide a shock automatically when a heart arrhythmia is detected.
NICE guidance supports new blood pressure device that can detect atrial...
New NICE medical technologies guidance published on 16 January 2013 supports the use of a device that can detect atrial fibrillation whilst blood pressure is being measured.
New clinical trial will use continuous cardiac monitoring to evaluate atrial...
Medtronic has announced the first patient implant for the REVEAL AF trial, which will evaluate the incidence of AF among patients suspected to be at a high-risk for the disease.
St Jude Medical announces FDA approval of new version of remote...
The newest version of Merlin.net provides clinicians with additional insight into changes in their patients' heart failure status through advanced impedance and lead monitoring capabilities.
Biotronik announces initiation of ProMRI clinical trial
The ProMRI trial will evaluate Biotronik's Entovis dual- and single-chamber pacemaker systems after exposure to magnetic resonance imaging at 30 US investigational centres.
First patient treated in trial to evaluate the safety and performance...
The ZERO AF study will include up to 33 sites in the USA, Europe and Asia-Pacific, and the results will be used to support a FDA submission for a paroxysmal AF indication.
St Jude Medical announces CE mark approval of ViewFlex Xtra ICE...
The new catheter provides real-time, high-resolution ultrasound images of blood flow and cardiac anatomy when paired with the ViewMate Z Intracardiac Ultrasound Console.
Philips showcases advanced imaging integration in electrophysiology at Boston AF Symposium
Philips provides a novel approach to real-time 3D imaging with improved workflow and collaborates with Biosense Webster to provide enhanced anatomical detail in the Biosense Carto 3 mapping system.
FDA clears FlexCath Advance Steerable Sheath for Arctic Front Advance Cryoballoon...
The FlexCath Advance Steerable Sheath is a new enhancement to the Arctic Front Advance Cryoballoon System, which is expected to facilitate easier access to the inferior veins when treating paroxysmal atrial fibrillation.
Over 1,000 atrial fibrillation cases performed with the HeartLight ablation system
CardioFocus has announced that more than 1,000 clinical cases have been performed worldwide using its HeartLight Endoscopic Ablation System technology for the treatment of atrial fibrillation.
First patient enrolled in MIRACLE EF clinical trial
The trial seeks to evaluate the effectiveness of cardiac resynchronisation therapy-pacemakers (CRT-Ps) in symptomatic heart failure patients with mild-to-moderate structural heart disease.
FDA approves apixaban to reduce the risk of stroke in AF...
On 28 December 2012, the FDA approved the anticlotting drug apixaban (Eliquis, Bristol-Myers Squibb), an oral tablet used to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
First of Nanostim’s leadless cardiac pacemaker systems implanted
Nanostim announces the implantation of its leadless pacemaker system in the LEADLESS study.
First patient implanted with the Boston Scientific INGEVITY pacing leads
Boston Scientific is beginning a clinical trial of patients implanted with the INGEVITY device.
Minimally invasive hybrid ablation is safe and effective for treatment for...
One year after treatment completion, 80% of patients in normal sinus rhythm without administration of drugs, study says.
Biotronik Home Monitoring reduces inappropriate ICD shocks by 52%
ECOST trial results published in European Heart Journal show the safety and efficacy of Home Monitoring.
Apixaban now available in the UK for the prevention of stroke...
The oral anticoagulant is a new prescription only treatment option for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation and one or more risk factors such as: prior stroke or transient ischaemic attack; age 75 years or older; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥ II).
European Journal of Heart Failure recognises research on vagus nerve stimulation...
BioControl Medical has announced that the pilot clinical study of its CardioFit vagus nerve stimulation system has been recognised as seminal original research in the European Journal of Heart Failure.
Dabigatran may be associated with better patient outcomes after a major...
Findings from the RE-LY trial and four other phase III trials report outcomes after a major bleed on dabigatran, despite the lack of a specific reversal agent, may be better than after a warfarin-associated bleed.
Do syncope units work?
Syncope is a very common medical problem with a yearly incidence of around 10 patients per 1,000 inhabitants. However, the rate of diagnosis when using a non-standardised approach is 42-54%. Mohamed Hamdan reviews the evidence for syncope units and whether they can be used to improve the rate of diagnosis of syncope.
Successful catheter ablation may reduce total mortality
A study recently published in EP Europace has shown that successful catheter ablation of atrial fibrillation may reduce the risk of total mortality, cardiovascular mortality and total vascular events in patients with an increased risk of stroke.
ECG screening has limited life-saving potential and would result in “staggering...
According to a study published in the Journal of the American College of Cardiology, a 20-year ECG screening programme of young athletes would only save 4,813 lives and would cost between US$51 billion and US$69 billion-resulting in enormous costs per life saved.
Nicolas Marsault appointed new chief financial officer of Endosense
Marsault joins the company as it continues to expand the commercial presence of its TactiCath contact-force sensing ablation catheter in Europe and prepares for the device's anticipated launch in the United States.
Sorin Group announces first Paradym RF implant with Smartview remote monitoring...
The Smartview remote monitoring system enables the patient's implantable device to communicate stored data wirelessly to the clinic where clinicians can review the information via a secure web application.
FDA clears Heart Monitor for iPhone
The Heart Monitor is initially intended for use by licensed medical professionals to record, display, store, transfer, and evaluate single-channel electrocardiogram rhythms.
First patient enrolled in BIO.Detect HF II study of CRT optimisation...
The intracardiac impedance sensor evaluates changes in impedance during the cycle of the heart and is expected to be a useful surrogate for several left ventricular haemodynamic parameters.
Mobile Cardiac Outpatient Telemetry helps to detect paroxysmal atrial fibrillation in...
A study published in the Journal of the Neurological Sciences has found that Mobile Cardiac Outpatient Telemetry (MCOT) supports the detection of a high rate of occult paroxysmal atrial fibrillation (PAF) in cryptogenic stroke and transient ischaemic attack (TIA) patients.
Assura implantable defibrillators get the CE mark approval
The Assura devices including the Quadra Assura CRT-D, the Unify Assura CRT-D and the Fortify Assura ICD, all from St Jude Medical, feature three new algorithms, more power and extended longevity.
Endosense secures US$40.3 million series c financing to advance cardiac arrhythmias...
Proceeds will be used to support commercialisation of the recently European introduction of the third-generation TactiCath Quartz force-sensing catheter; development of next generation products; completion of the TOCCASTAR investigational device exemption clinical study follow-up and FDA approval submission for the TactiCath.
HeartSine Samaritan public access defibrillator 300/300P shows battery problems
HeartSine has notified that certain Samaritan 300/300P public access defibrillators (PAD) have been found to intermittently turn on and off, which may eventually deplete the battery.
Early data for over-the-wire mesh ablation system is “encouraging”
At Europe AF (18-19 November, London, UK), Dirk Mueller, Bad Bevensen, Germany said that the initial results of the GENESIS study validated the usability of the Over-the-Wire Mesh Ablation System (OTW MAS, Encompass, Bard Electrophysiology) for paroxysmal atrial fibrillation.
Undiagnosed sleep-disordered breathing is common but does not predict mortality in...
Wolfram Grimm, Baldingerstraβe, Germany, and others reported in EP Europace that while undiagnosed sleep-disordered breathing is common in patients with implantable cardioverter defibrillators (ICD) and chronic heart failure, it does not independently predict appropriate ICD therapy or mortality.
European Commission approves apixaban for prevention of stroke and systemic embolism...
The European approval for apixaban is supported by the pivotal phase 3 trials ARISTOTLE and AVERROES, which evaluated approximately 24,000 patients with non-valvular atrial fibrillation.
First 34 US patients implanted with Evia HF-T pacemaker after FDA...
Evia HF-T offers Biotronik's Closed Loop Stimulation (CLS) technology. The company states that this is "the only rate response algorithm that is FDA-approved to respond to exercise and acute mental stress on a beat-to-beat basis."
Boston Scientific defibrillators receive the CE mark approval for extended longevity
Boston Scientific has received the CE mark approval for increased longevity projections for the Incepta, Energen, Punctua, Cognis and Teligen implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds).
High-dose visually guided laser ablation superior to low-dose ablation in treatment...
New data have shown that acute pulmonary vein isolation was achieved after a single visually guided circular lesion set in 89% and 69% of patients in the high and low-dose groups, respectively.
Philips to provide defibrillators on KLM and Air France’s passenger flights
According to a company release, the HeartStart FRx AED has been tested and certified for airline use and does not interfere with airplane electronics.
HeartLight Ablation System receives unique classification code in Germany for the...
Use of the new code allows the German Institute of Medical Documentation and Information to track the number of atrial fibrillation patients being treated with laser ablation guided by endovascular endoscopy and provides the initial step to receiving a specific diagnosis-related group code for the procedure.
Durata and Riata ST Optim leads show low rates of insulation...
The Population Health Research Institute analysed data from three actively monitored registries: the OPTIMUM, SCORE and SJ4 Post-Approval registries, all sponsored by St Jude Medical. The combined data from these registries currently represents 10,987 leads implanted at 293 sites.
Dabigatran demonstrates sustained positive outcomes for over four years, RELY-ABLE study...
New data from the RELY-ABLE study have provided additional support to the safety profile and efficacy of dabigatran etexilate (Pradaxa, Boehringer Ingelheim) for stroke prevention in patients with non-valvular atrial fibrillation.
A smart way to make community screening for atrial fibrillation feasible?
Jerrett Lau, Concord Hospital, Sydney, Australia, and others reported, at the American Heart Association (AHA) scientific sessions, that a smartphone app could be used to diagnose atrial fibrillation in a community setting.
Can heartbeat-induced vibrations be used to power pacemakers?
Piezoelectric harvesters could be used to convert heartbeat-induced vibrations into enough electrical energy to power a cardiac implantable electronic device, according to a preliminary study presented by M Amin Karami, Ann Arbor, USA, at the scientific sessions of the American Heart Association in Los Angeles.
MADIT-RIT trial shows improved device programming reduces inappropriate therapy and risk...
Data presented at the American Heart Association Scientific Sessions have shown that improved programming of dual-chamber implantable cardioverter defibrillators or cardiac resynchronisation therapy defibrillators can reduce inappropriate therapy by up to nearly 80% and increase patient survival by up to 55%.
Better patient outcomes gained from biventricular pacing in patients with AV...
Data presented at the American Heart Association's 2012 Scientific Sessions show 26% relative risk reduction in the composite of death, healthcare utilisation visits requiring IV heart failure therapy, and significant increase in left ventricular end systolic volume index among the patients receiving biventricular pacing.
Bleeding rates with dabigatran are not higher than bleeding rates with...
The assessment is consistent with observations from the large RE-LY clinical trial used to approve dabigatran (Pradaxa) for the prevention of stroke in patients with non-valvular atrial fibrillation.
Gold-tip ablation catheters create lesions 33% faster than platinum-iridium-tip catheters
Results from the GOLDEN TIME study have shown that gold-tip radiofrequency ablation catheters enable faster, more efficient ablation procedures than platinum-iridium-tip catheters.
Michael H Carrel new president and CEO of AtriCure
Michael H Carrel was most recently the president and CEO of Vital Images. Under his leadership at Vital Images, the company grew revenue and profitability, increased global market share, expanded its presence to over 90 countries and raised US$100 million in equity financing.
Lancet Series explores latest developments in cardiac arrhythmia
A new Lancet Series explores the latest developments in the diagnosis, treatment and biology of cardiac arrhythmias, ahead of the American Heart Association's annual meeting (Los Angeles, USA, November 3-7).
“Implantable devices are not a luxury”
Implantable devices for treating cardiac arrhythmias, which include ICD's, are underused in parts of Europe. Conclusions of the ICD for Life Summit (Belgrade, Serbia, 19-20 October).
Percutaneous left atrial appendage suture closure can be performed effectively, study...
Krzysztof Bartus, Krakow, Poland, and colleagues have reported that left atrial appendage closure for stroke prevention in patients with atrial fibrillation using a percutaneous ligation approach can be performed effectively and is associated with a low rate of complications.
Reused ICDs function normally without an increased risk of complications
Behzad Pavri, Philadelphia, USA, and colleagues have found that implantable cardioverter defibrillators (ICDs) with three or more years of estimated remaining battery life can be reused, delivering life-saving therapy without increasing the risk of complications.
Is “parasympathetic withdrawal” a critical factor in heart failure progression?
Randy Lieberman, director of electrophysiology at Harper University Hospital: CardioVascular Institute, Detroit Medical Center, Detroit, USA, reviews the role of the parasympathetic nervous system in heart failure.
Medtronic launches Advisa DR MRI SureScan pacing system in Japan
With the Advisa MRI system, pacemaker patients in Japan will have access to full body scans, without positioning limitations in the magnetic resonance imaging (MRI) scanner.
nContact receives US patents for epicardial ablation technology for cardiac arrhythmias
The four patents issued cover essential components of the multidisciplinary epicardial-endocardial approach pioneered by nContact for the treatment of cardiac arrhythmias.
Biotronik launches BioMonitor implantable cardiac device in Europe
The device is a subcutaneous implantable leadless cardiac monitor for the long-term continuous remote monitoring of patients with arrhythmias such as atrial fibrillation (AF), bradycardia, sudden rate drop, asystole and tachycardia.
Majority of Americans unaware of effective treatment options to protect against...
The survey has shown that 75% of Americans are unaware that an implantable cardioverter defibrillator (ICD) is an effective treatment option to protect those at risk of SCA.
Successful results with HeartLight presented at Netherlands Society of Cardiology Congress
Data show high acute success and freedom from atrial fibrillation at six months follow-up.
Boston Scientific to acquire Rhythmia Medical
Rhythmia Medical is a developer of next-generation mapping and navigation solutions for use in cardiac catheter ablations and other electrophysiology procedures, including atrial fibrillation and atrial flutter.
Moderate alcohol consumption may increase risk of atrial fibrillation
Yan Liang, Beijing, China, and colleagues have found that moderate alcohol consumption may increase the risk of atrial fibrillation among people with existing cardiovascular disease and that binge drinking further increases this risk. The researchers analysed data from 30,433 patients enrolled in the ONTARGET and TRANSCEND trials.
Catheter ablation can be performed safely while a patient is on...
Stephen Page and Richard Schilling, St Bartholomew's Hospital, UK, explain why catheter ablation can be performed safely in most patients with uninterrupted warfarin.
Inappropriate programming most common cause of sustained loss of CRT pacing
A study, published ahead of print in Circulation Arrhythmia and Electrophysiology, has found that inappropriate programmed atrioventricular delays was the most common cause of sustained loss of CRT pacing.
Bionet America introduces new ECG Cardio7
The Cardio7 is a 12 channel interpretive resting ECG with a 7" hi-resolution color TFT LCD touch screen.
FDA acknowledges receipt of resubmission of apixaban’s new drug application
The new drug application for apixaban (Eliquis / Bristol-Myers Squibb and Pfizer) is intended to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
FDA approves first subcutaneous implantable defibrillator for patients at risk of...
The S-ICD System (Boston Scientific) sits below the skin without the need of leads to be placed into the heart. This leaves the heart and blood vessels untouched, offering patients an alternative to transvenous implantable cardioverter defibrillators (ICDs).
Biotronik launches new online Travel Guide for patients with Biotronik cardiac...
The Travel Guide, which is available in English, German, French, Italian, Dutch, Portuguese and Spanish, gives patients information about recommended hospitals and medical centres in more than 100 countries around the globe.
New consensus statement on CRT unveiled at Cardiostim
Cardiac Rhythm News talked to Jean-Claude Daubert, who is one of the founders of the European Heart Rhythm Association (EHRA), about key details of the new consensus statement on cardiac resynchronisation therapy (CRT) device implantation and follow-up.
Boston Scientific launches new implantable defibrillation lead in Europe and Asia
The Reliance 4-Front lead is designed to streamline surgical procedure for treatment of heart failure and sudden cardiac arrest.
Apixaban receives CHMP positive opinion for the prevention of stroke and...
The positive opinion from the European Committee for Medicinal Products for Human Use (CHMP) was based on the pivotal ARISTOTLE and AVERROES studies, which evaluated apixaban in approximately 24,000 patients with non-valvular atrial fibrillation.
IMPROVE Brady study of common arrhythmias enrols first patient
The IMPROVE Brady (Registry to improve the adoption of consensus treatment guidelines) trial will evaluate the impact of physician led improvement initiatives on the diagnosis and treatment of patients with a sinus node dysfunction.
Are infants with supraventricular tachycardia being over treated?
A study by Shubbhayan Sanatani, Vancouver, Canada, and colleagues has shown that infants (aged 0-1 year) with supraventricular tachycardia who receive medical prophylaxis do not have a first recurrence after six months of age-suggesting that the current approach of treating patients for the first year of life is too long.
Should we offer screening for Brugada syndrome in children?
Juan Pablo Kaski, London, UK, summarised for Cardiac Rhythm News the arguments, for and against, screening for Brugada syndrome in children.
Anne Gillis
Anne Gillis, current president of the Heart Rhythm Society (HRS), talked to Cardiac Rhythm News about her career achievements, her plans for the HRS and the role of the society to encourage women into electrophysiology.
TYRX receives New Product Innovation Award for antibacterial envelope
The AIGISRx Antibacterial Envelope helps to reduce infection by delivering antibiotics directly to the surgical site in the first 7 to 10 days following implantation of cardiovascular implantable electronic devices.
Sorin receives approval and announces launch of Reply and Esprit families...
Both families of devices include easy-to-use functions and patient safety features, with Reply, at 8ccs, maintaining its position as the world's smallest dual-chamber pacemaker.
AtriCure launches three-year post-approval study for the surgical correction of atrial...
The 350-patient study is a follow-up to the company's 14 December 2011 FDA approval of the AtriCure Synergy Ablation System for the surgical treatment of atrial fibrillation.
New HeartLight data reinforce single procedure chronic success rates in the...
Results presented at the ESC congress showed that pulmonary vein isolation with the device led to a 99% acute success rate and up to an 82% chronic success rate at 12 months follow-up.
Preventice receives FDA clearance to market BodyGuardian Remote Monitoring System
Developed in collaboration with Mayo Clinic, the BodyGuardian System uses sophisticated algorithms to support remote monitoring for individuals with cardiac arrhythmias.
EPIC Alliance of female electrophysiologists session highlights at ESC
Sabine Ernst, Silvia G Priori, Andrea Russo, among other well-known female electrophysiologists presented research focused on gender specific topics at the ESC Congress in Munich, Germany.
Updated guidelines for device-based therapy of cardiac rhythm abnormalities released
The American College of Cardiology Foundation, American Heart Association and the Heart Rhythm Society jointly released updated guidelines for their use in treating heart rhythm disorders.
Estech launches the Cobra Fusion system for cardiac ablation
Cobra Fusion incorporates proprietary Versapolar technology which delivers both bipolar and monopolar radiofrequency (RF) energy to the targeted cardiac tissue, enabling transmural lesion formation in thin and thick cardiac tissue.
Health Canada approves the HeartCheck ECG PEN
According to CardioComm Solutions, the HeartCheck ECG PEN takes accurate heart readings anytime in only 30 seconds.
Should female gender be included in risk scores for stroke?
Female gender is only a significant risk for stroke in patients with atrial fibrillation among those aged ≥75 years-indicating its inclusion in risk scores for stroke may not be required, according to study presented at the ESC annual meeting.
New AF update calls for greater focus on identifying “truly low...
In a focused update of its 2010 guidelines for the management of atrial fibrillation, the European Society of Cardiology (ESC) "strongly recommends" a practice shift towards initial identification of patients at "truly low risk" of stroke. Gregory YH Lip, Birmingham, UK, presented the update.
PARCADIA study starts patient enrolment
The PARCADIA study aims to identify risk factors that can help predict appropriate implantable cardioverter defibrillator (ICD) interventions in patients with ischaemic cardiomyopathy who have received an ICD for primary prevention according to the current European Society of Cardiology guidelines.
GARFIELD baseline data presented at ESC
Baseline data from the first cohort of the GARFIELD registry (Global anticoagulant registry in the field) shows that established, evidence-based guidelines for preventing stroke in patients with atrial fibrillation (AF) are not being followed in many patients and across diverse populations.
“Clear gap” between guidelines and clinical practice
Elena Arbelo, Barcelona, Spain, presented one-year results from the first European study of real-life epidemiology of catheter ablation for atrial fibrillation. At the ESC annual meeting, Arbelo said: "There is a clear gap between recommendations and actual clinical practice."
Medtronic receives FDA approval, CE mark for Arctic Front Advance Cardiac...
A company release states that this second-generation system provides a more efficient approach to treating paroxysmal atrial fibrillation than point-by-point, radiofrequency ablation.
Obesity increases the risk of atrial fibrillation in fertile women
A study presented at the ESC annual meeting (25-29 August 2012) suggests that obesity increases the risk of atrial fibrillation in women of child-bearing age, indicating that lifestyles measures could be used to prevent the development of atrial fibrillation in this population.
CRT consensus set to standardise and improve care for patients worldwide
EHRA and the HRS have published a joint consensus statement-for the first time-on cardiac resynchronisation therapy
Female gender increases stroke risk in older AF patients by 20%
According to a study presented at the annual meeting of the European Society of Cardiology (ESC), 25-29 August, Munich, Germany, being female increases the risk of stroke in patients with atrial fibrillation who are >75 years by 20%
Dabigatran may be more effective in the long term than rivaroxaban...
The analysis, based on two large scale trials including more than 32.000 patients combined, suggests that patients treated with dabigatran may have lower rates of ischaemic stroke and intracranial haemorrhage, and also accumulate lower costs from acute care and long-term follow-up over their lifetime than patients treated with rivaroxaban.
Watchman Left Atrial Appendage Closure Device receives CE mark approval for...
The Watchman device (Boston Scientific) is indicated to be used in patients with atrial fibrillation and a contraindication to warfarin and the newer oral anticoagulants.
First heart failure patient treated in the UK with novel nerve-stimulation...
This first procedure is part of the INOVATE-HF study, a clinical trial designed to study the safety and efficacy of the CardioFit system to reduce hospitalisation and death among patients with heart failure.
Intermittent rhythm monitoring fails to identify atrial fibrillation recurrence in a...
Efstratios Charitos, Luebeck, Germany, and colleagues have reported in Circulation that two to four sessions of 24h intermittent rhythm monitoring has been shown to "under-detect atrial fibrillation recurrence and thus overestimate success rates of therapeutic interventions".
New insights into the basics of arrhythmias
Irene Savelieva and John Camm report on a session, which was co-chaired by Savelieva, at Cardiostim 2012 (13-16 June, Nice, France) about the latest information on the pathophysiology of arrhythmias.
Rivaroxaban to be explored in two new studies
X-VeRT and VENTURE-AF will evaluate rivaroxaban (Xarelto, Bayer HealthCare) in patients with non-valvular atrial fibrillation undergoing cardioversion or ablation therapy.
HeartLight Endoscopic Ablation System now available in Australia
Monash Medical Centre in Melbourne is the first cardiology centre in the country to offer this catheter ablation treatment.
Viva/Brava cardiac resynchronisation therapy devices get CE mark
Medtronic's Viva/Brava cardiac resynchronisation therapy devices (CRT-D) feature a new algorithm, called AdaptivCRT, which significantly improves heart failure patients' response rate to CRT-D therapy, as compared to historical CRT trials.
Spouses of people suffering a heart attack need care for increased...
Spouses of people who suffer a sudden heart attack have an increased risk of depression, anxiety, or suicide after the event, even if their partner survives, according to new research published in the online August issue of the European Heart Journal.
PINNACLE-AF registry shows early patterns for new atrial fibrillation treatments
The American College of Cardiology (ACC) has released first findings from its expanded outpatient registry, the PINNACLE registry, focusing on atrial fibrillation and including the next generation of anticoagulants.
Uninterrupted warfarin is associated with a lower risk of bleeding than...
A meta-analysis, published in the American Journal of Cardiology, indicates that compared with a heparin-based bridging therapy, uninterrupted warfarin throughout surgery for implantation of a cardiac implantable electronic device (CIED) is associated with a decreased risk of postoperative bleeding and similar risk of thromboembolic events.
Non-invasive cardiac output monitor effective in the trauma setting, study shows
The Nicom (Cheetah Medical) haemodynamic monitoring system provides greater insight on patient status in the trauma setting and leads to a significant decrease in patients' length of hospital stay.
FDA recommends continuous monitoring of patients implanted with Riata or Riata...
The recommendation is due to an increase in frequency of reported premature Riata insulation failures.
Medtronic launches CareLink Express Service
This service is a remote monitoring system that enables clinicians in healthcare facilities to quickly obtain data regarding the status of Medtronic implanted cardiac devices, facilitating faster treatment decisions.
First US patient implanted with Lumax 740 dual-chamber ICD
The Lumax 740 defibrillator (Biotronik) differentiates atrial from ventricular arrhythmias to avoid unnecessary shocks.
Indirect comparison of new anticoagulants provides insight into how the drugs...
Gregory Lip and colleagues have made an indirect comparison of the new anticoagulants. They have found "no profound significant differences" in efficacy between apixaban (Eliquis, Pfizer and Bristol-Myers Squibb) and both does of dabigatran (Pradaxa, Boehringer Ingelheim) and Rivaroxaban (Xarelto, Bayer).
Biosense Webster launches Carto 3 MEM version for mapping arrhythmias
The new Carto 3 Multi-Electrode Mapping (MEM) version provides a fast method for mapping and diagnosing arrhythmias, while using sensor-based MEM-enabled catheters to maintain a high degree of anatomical accuracy.
Novel coating fails to prevent lead abrasions
A preliminary study, by Robert Hauser and others, published in EP Europace, indicates that a silicone-polyurethane copolymer (Optim, St Jude Medical) designed to prevent implantable cardioverter defibrillator (ICD) lead insulation abrasions fails to prevent such abrasions.
First-ever consensus statement on pacemaker device and mode selection
In a joint consensus statement, the Heart Rhythm Society (HRS) and the American College of Cardiology Foundation (ACCF) have issued recommendations, in collaboration with the Society of Thoracic Surgeons, on the use of single- vs. dual-chamber devices for patients who are eligible (according to existing guidelines) for pacemaker implantation.
ICDs have contributed to the reduction in ventricular fibrillation out of...
Researchers in The Netherlands have found that the use of implantable cardioverter defibrillators (ICDs) may be partly responsible for the reduction in the number of ventricular fibrillation out of hospital cardiac arrests.
CardioNet to acquire Cardiocore
The total consideration to be paid by CardioNet will be US$23.5 million. The transaction, subject to customary closing conditions, is expected to close in the third quarter of 2012.
Ingenio and Advantio pacemakers receive CE mark approval for use in...
With the Image Ready technology, Ingenio MRI pacemakers, in combination with Fineline II leads, allow patients to undergo MRI procedures as needed.
HRS and the American College of Cardiology Foundation release expert consensus...
International consensus writing group provides specific recommendations on mode choices for single- versus dual- chamber devices for adult population.
First CRT-D patient enrolled in the EuroEco ICD/CRT-D trial assessing the...
The aim of the study is to assess the economic effects of Biotronik Home Monitoring based remote follow-up management compared to conventional in-clinic or practice follow-ups.
St Jude Medical introduces Japanese launch of Unify Quadra cardiac resynchronisation...
The Unify Quadra CRT-D system was designed to help the heart perform in its most natural state by synchronising the left and right ventricles of the heart through timed electrical pulses.
St Jude Medical announces initial findings from Riata Lead Evaluation Study
Initial phase-one results of the St Jude Medical's Riata Lead Evaluation study have found that externalised conductors occurred in 9.3% of the smaller-diameter Riata ST 7F leads, and in 24% of the larger-diameter Riata 8F leads.
Biocontrol Medical gets FDA approval to begin second phase of INOVATE-HF...
CardioFit is an implantable electrical stimulation device designed to improve heart function in patients with congestive heart failure.
ViewFlex Xtra Intracardiac Echocardiography catheter gets FDA clearance
ViewFlex Xtra Intracardiac Echocardiography catheter (St Jude Medical) is used to treat arrhythmias with radiofrequency ablation and to close atrial septal defects with structural heart procedures.
SPIRIT-ICD study patient enrolment completed
The SPIRIT-ICD study explores benefits of intensified diagnostic follow-up and timely therapeutic intervention for high-risk patient group receiving ICD therapy.
Does the hybrid procedure have the potential to treat atrial fibrillation...
The hybrid procedure, which combines a transvenous endocardial and a thoracoscopic epicardial approach in a single ablation procedure, is associated with a 83% success rate at one year in patients with atrial fibrillation, a study has found.
Six baseline factors may predict “super response” to cardiac resynchronisation therapy
Data from the MADIT-CRT study indicates that there are six baseline factors that predict left ventricular ejection fraction (LVEF) "super response" in patients with a cardiac resynchronisation therapy with defibrillator (CRT-D) device, report Jonathan Hsu and colleagues.
Patient enrolment in PREVAIL study for Watchman device completed
The PREVAIL study is designed to gain FDA approval for the Watchman Left Atrial Appendage (LAA) Closure device (Boston Scientific).
Heart Rhythm Society named Bronze winner at the 33rd Annual Telly...
The Sudden Cardiac Arrest Patient Education DVD is the Society's first-ever educational DVD developed as a resource specifically for patients at risk of sudden cardiac arrest and part of the Society's annual SCA awareness initiatives.
Experts call for better management of atrial fibrillation
A new report launched on 3 July written by UK experts shows that atrial fibrillation, a condition that affects up to 1 million people in the UK, is not being optimally managed.
Philips announces CX50 xMATRIX echocardiography system
According to the company, this is the world's first portable echocardiography system to offer real time 3D Transesophageal Echo (Live 3D TEE), which enables 3D visualisation of the cardiac structure and function as well as new perspectives of the heart in real time.
Sorin Group announces the acquisition of California Medical Laboratories
California Medical Laboratories develop, manufacture and distribute a complete range of cannulae, catheters and accessories for cardiac surgery.
Ondansetron may pre-dispose patients to develop Torsades de Pointes, FDA warns
Results from a recently completed clinical study suggest that a 32mg single intravenous dose of ondansetron (Zofran, GlaxoSmithKline) may affect the electrical activity of the heart, which could pre-dispose patients to develop Torsades de Pointes.
Pregnancy in women with implantable cardioverter defibrillators associated with a good...
Pia Schuler, The Grown Up Congenital Heart Disease Unit, The Heart Hospital, University College London Hospitals, London, UK, and others reported that there is little data for the effect of pregnancy on women with ICDs. Thus, they reviewed the outcome of pregnant women with an ICD at their centre.
FDA responds letter for apixaban’s new drug application
Bristol-Myers Squibb and Pfizer announced that the FDA has issued a complete response letter regarding the new drug application for apixaban (Eliquis) for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
St Jude Medical comments on MAUDE database report about Durata Lead
St Jude Medical states: Through our investigation of the MAUDE database report submitted to the FDA on 2 May 2012, and information provided to the company by the FDA, including model number, implant and event dates, St Jude Medical has identified a single Durata lead that matches the available information.
Rhythm control may be superior in the long term
A study published in the Archives of Internal Medicine indicates that in the long-term, rhythm control for atrial fibrillation may be associated with lower mortality than rate control.
Global and inferior J wave amplitudes associated with recurrent ventricular fibrillation
According to a study presented by Laurent Roten, Bordeaux, France, at Cardiostim, in patients with early repolarisation and who had survived a sudden cardiac arrest, global and inferior J wave amplitudes were significantly higher in patients with recurrent ventricular fibrillation than in the remaining patients.
Much debate over device infection study at Cardiostim
A study, which suggested that a novel therapy for managing cardiovascular implantable electronic device (CIED) infections could markedly diminish the need for device removal, sparked a lively discussion at Cardiostim (13-16 June, Nice, France).
Personalised medicine in cardiac arrhythmia management: Hype or hope?
Stefan Kääb outlines the different steps to developing comprehensive personalised medicine strategies in the context of cardiac arrhythmias and reviews what essential elements such strategies require.
New ICD algorithm could inhibit inappropriate therapy
Scott Beau and colleagues outlined a new implantable cardioverter defibrillator (ICD) algorithm that could be used to discriminate ventricular tachycardia/ventricular fibrillation noise from pace/sense conducted lead noise, potentially reducing inappropriate therapy due to lead noise.
Ambu completes acquisition of Unomedical’s electrode and diathermy business
Unilect, Neutralect, and Biotab electrodes are now available alongside Ambu Blue Sensor and Ambu Neuroline.
Flec-SL trial proves efficacy of short-term antiarrhythmic drug treatment
Short-term antiarrhythmic drug treatment after cardioversion is nearly as effective in preventing recurrences of atrial fibrillation as long-term treatment, data from Flec-SL show.
European perspective on the RCPE guidelines
John Camm and Karl-Heinz Kuck, on behalf of the European Heart Rhythm Association (EHRA), review the recent consensus document from the Royal College of Physicians of Edinburgh (RCPE) on the management of atrial fibrillation.
TactiCath Quartz Force-Sensing Ablation Catheter gets CE mark approval
Endosense and Biotronik have announced that Endosense's TactiCath Quartz force-sensing ablation catheter has been CE mark approved in Europe.
Sorin Group and Orange Business Services launch remote monitoring solution in...
Using the Smartview remote monitoring solution, healthcare providers can now access valuable cardiac data and alert messages from Sorin's implantable Paradym RF devices, while the patient is at home.
Chu-Pak Lau
Chu-Pak Lau, honorary clinical professor, Division of Cardiology, Department of Medicine, University of Hong Kong, Queen Mary Hospital, Hong Kong, talked to Cardiac Rhythm News about rate-responsive pacemakers, the potential role of gene therapy in managing atrial fibrillation, and leadless pacing.
First patient active with Latitude NXT Remote Patient Management System
The system offers wireless and automatic remote follow-up and optional integrated cellular GSM connectivity.
ESC-EHRA and Cardiostim-Reed to hold a joint annual congress
The European Heart Rhythm Association (EHRA) a registered branch of the European Society of Cardiology (ESC) and Cardiostim"Reed have agreed to hold a joint annual congress during 2014"2017.
EPIC Alliance addresses hot topics for female electro-physiologists at Cardiostim 2012
The session will include electrophysiology-related presentations made by leading female physicians and researchers from around the world. Topics will include "Atrial fibrillation ablation: beyond pulmonary vein isolation" and "Controversies in lead extractions for noninfectious indications."
Philips launches SaveLives.net
The Save Lives website is an online campaign to inform people about sudden cardiac arrest and to empower them to act.
Zoll to showcase its LifeVest wearable defibrillator at Cardiostim
Zoll, manufacturer of the LifeVest wearable defibrillator and the RecueNet Medgate 12-Lead management solution, will display its products at Cardiostim (13-16 June, Nice, France) this week
Boston Scientific acquires world’s first subcutaneous ICD
Boston Scientific has added the first and only, commercially available, subcutaneous implantable cardioverter defibrillator technology to its product portfolio
Study confirms the value of Cambridge Heart’s MTWA test as a...
At a median follow-up time of 22 months, patients with an abnormal MTWA test were 11 times more likely to experience a major arrhythmic cardiac event than patients with a normal MTWA result.
Karl-Heinz Kuck to chair Topera Medical’s International Scientific Advisory Board
Karl-Heinz Kuck is currently director of Cardiology at the Asklepios Klinik St Georg in Hamburg, Germany. He has authored more than 450 scientific papers, has served as a board member of the European Heart Rhythm Association (EHRA) for the past four years and is the current EHRA president-elect.
Convergent procedure building support for interdisciplinary approach to atrial fibrillation
An article in the Journal of Cardiovascular Electrophysiology outlines the development and results of the new interdisciplinary procedure, the convergent procedure
Stereotaxis appoints William C Mills III as chairman of board of...
Mills replaces Fred A Middleton, who will remain on the board as an independent director.
Stepping beyond ECG: Electrocardiographic imaging
Yoram Rudy, St Louis, USA, talked to Cardiac Rhythm News about the novel imaging technique, electrocardiographic imaging, which was developed in his laboratory.