Hans Kottkamp, medical director of the Electrophysiology Department, Hirslanden Hospital Zurich, Switzerland, presented a new device strategy that combines diagnostic and treatment capabilities for atrial fibrillation in one system, at the AF Symposium in Orlando USA (9–11 January 2014). He spoke to Cardiac Rhythm News about the combined system.
What are the features of this new mapping and ablation system?
The Globe Mapping and Ablation system [from Kardium] is a complete system intended for mapping and treatment (ablation) of atrial fibrillation.
The system consists of the Globe catheter, a software console and an electronics controller including a radiofrequency (RF) generator.
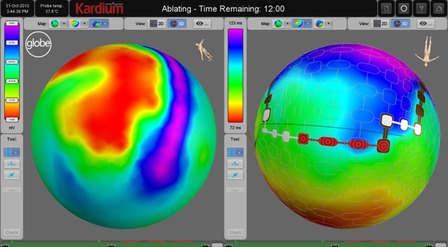
It contains an expanding, contacting, semi-compliant array of up to 275 flat electrodes that expand to contact the entire inside of the left atrium. Once deployed into the atrium, the array does not move, ensuring good and stable contact between the electrodes and the atrial wall. The array can then: map anatomy, map electrical activity, measure temperature, pace and ablate using RF energy.
The Globe software console uses the high density array of electrodes to provide detailed electrophysiological mapping. With up to 275 contacting electrodes, it is possible to do activation mapping and voltage mapping quickly and easily for a single cardiac cycle.
In addition, the Globe Wave maps show real time propagation of electrical activity across the atrium, even during ablation.
Using the software console, ablation sites are selected on the map, and then the selected electrodes are automatically activated by the system, and allow for up to 16 simultaneous ablations.
What differentiates this system from others in the market?
The system has been designed with a global approach in mind, since it is capable of both detailed mapping plus ablation.
The high density of fully contacting electrodes allows for much more detailed global mapping of the atrium than before (activation mapping, voltage mapping of the substrate, Globe Wave maps). The Globe system provides simultaneous multi-electrode mapping.
The system uses a temperature controlled setpoint, with precise temperature measurement and low power (5-10W) to control the ablation. The electrodes heat only tissue, not blood, meaning there is no requirement for irrigation. In addition, the stable and complete contact during ablation provides a reproducible and predictable electrogram response to the ablation. This stable contact also results in predictable and repeatable tissue effects (ablation lesions) the “gentle ablation”.
Do you have any supporting pre-clinical data on the device?
We have done extensive pre-clinical in-vivo and in-vitro testing to prepare for the trial, where we will gather clinical data.
With this fully contacting flat array electrodes, we were able to show a clear stepwise increase in lesion depth depending on the preset temperature. In addition, a clear stepwise increase was found depending on energy application duration. This is in contrast to “conventional” ablation, where no reliable relationship between catheter tip temperature measurements and tissue effects can be found in-vivo due to the partial and non-stable contact of the ablating electrode with the target tissue.
In the pre-clinical in-vivo testing, we could perform circumferential pulmonary vein isolation. In addition, pulmonary vein entrance block as well as exit block could be confirmed on-line during “Globe Wave mapping”. Importantly, the electrophysiologist is not standing at the catheter table with this device during ablation, but he/she is sitting at the software console, selecting the electrodes for ablation and observing/controlling the effects during the therapeutic procedure.
When are you expecting to start using the device in humans?
We will be starting clinical trials in Europe later this year. We are planning to enroll 60 patients with paroxysmal and persistent atrial fibrillation in four centres.












