Abbott has announced a collaboration with AtaCor Medical to advance a next-generation investigational extravascular implantable cardioverter defibrillator (EV-ICD) system designed to deliver defibrillation therapy to people with life-threatening heart rhythms.
Abbott has announced a collaboration with AtaCor Medical to advance a next-generation investigational extravascular implantable cardioverter defibrillator (EV-ICD) system designed to deliver defibrillation therapy to people with life-threatening heart rhythms.
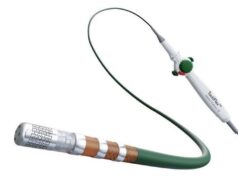
AtaCor is a cardiac rhythm management company developing extravascular defibrillation technologies designed to reduce risks associated with traditional ICDs. Through the collaboration, AtaCor’s investigational parasternal EV-ICD lead, Atala, will be paired with Abbott’s investigational ICD system.
According to a company press release, the Abbott-AtaCor investigational EV-ICD system is a minimally invasive approach that combines the benefits of traditional ICDs with innovative design improvements, avoiding complications like vascular injury, lead fractures or malfunctions, and lead infections. By keeping lead components outside the heart and the vasculature, the approach expects to address longstanding lead management considerations, and may reduce the need for complex revisions associated with leads placed through veins and across cardiac structures, the release also states.
As part of the collaboration, AtaCor plans to initiate a pivotal investigational device exemption (IDE) clinical trial—the ALARION EV study—in 2026 to evaluate the AtaCor and Abbott investigational parasternal EV-ICD system. AtaCor’s Atala lead remains outside of the blood vessels and is placed into the body through a rib space adjacent to the left side of the breastbone. In addition to delivering defibrillation shocks, the novel directional lead design is intended to deliver pacing energy toward the heart more efficiently than currently available products, Abbott claims.
“Abbott is committed to advancing transformative therapies in cardiac rhythm management,” said Randel Woodgrift, senior vice president of Abbott’s cardiac rhythm management business. “From Abbott’s revolutionary Aveir leadless pacemaker to advances in conduction system pacing technologies, and now the potential of the next-generation EV-ICD, Abbott is redefining what’s possible to improve patient care worldwide.”
“The Abbott-AtaCor investigational system represents an exciting step forward in cardiac care,” added Paul Friedman (Mayo Clinic, Rochester, USA). “Its ease of implantation and potential to enable ICD therapy outside the heart while maintaining contact with cardiac tissue stands to offer an important novel therapeutic option for patients in need of life-saving protection.”