Tag: ICD
Impulse Dynamics announces first implantation of combined CCM and ICD device
Impulse Dynamics has today announced the completion of the first implantation for the INTEGRA-D clinical trial, designed to evaluate the safety and efficacy of...
Study results demonstrate high detection accuracy for atrial fibrillation using single-lead...
Results from the MATRIX study show that the high detection accuracy of DX single-lead implantable cardioverter defibrillator (ICD) systems for atrial fibrillation (AF) episodes...
Researchers warn of safety risk to ICDs from mobile devices
Portable electronic devices containing magnets may inhibit pulse generators for implanted cardiac devices (ICDs) and pacemakers according to new research published in Circulation: Arrhythmia...
Sex and race disparities found in ICD interventions for cardiac patients
Results of a clinical trial show stark disparities in the use of life-saving implantable cardioverter defibrillator (ICD) interventions based on sex and race, suggesting...
Abbott receives CE mark approval for Gallant ICD and CRT-D devices
Abbott has announced that it has received CE mark approval for the Gallant implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) devices.
The...
One in three patients treated in line with ICD guidelines
A study of patients undergoing implantable cardioverter defibrillator (ICD) therapy across three centres, found that only one in three were treated in concordance with...
Patients with implantable cardioverter defibrillators do not adhere to driving restrictions
Jenny Bjerre outlines the results of a study presented at the European Society of Cardiology Congress (ESC 2019; 31 August–4 September, Paris, France) which...
US physicians now treating patients with Biotronik’s Acticor DX devices
Biotronik recently announced the full commercial launch of the Acticor device family, including Acticor DX and CRT-DX devices. Leading electrophysiologists throughout the USA are...
“No serious adverse outcomes” with ICD shocks in young athletes playing...
A post-hoc analysis of the ICD Sports Registry—which has already indicated that many patients with implantable cardioverter defibrillators (ICD) can safely participate in vigorous...
Medtronic begins pilot study of investigational extravascular ICD system
Medtronic plc have announced the start of a pilot study of its investigational extravascular implantable cardioverter defibrillator (EV ICD) system, in which a lead...
Battery performance alert and cybersecurity firmware updates for certain Abbott implantable...
The US FDA has approved a firmware update that is now available and is intended as a corrective action (recall), to reduce the risk...
Murj expands offering to manage in-office cardiac device monitoring
Murj, a healthcare technology company focused on transforming implantable cardiac device management, has announced the availability of OnSite— the industry’s first cloud-based workflow solution...
Socioeconomic status and geographic region affect patient access to ICDs in...
There is a disparity between indications for and utilisation of implantable cardioverter defibrillators (ICDs) in Asian patients with heart failure, a recent study finds....
Boston Scientific gets FDA approval for MRI labelling and launches Resonate...
Boston Scientific has launched the Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) systems featuring the HeartLogic Heart Failure...
FDA approval for MRI-compatibility for Ellipse ICD
Abbott has received US Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labelling for one of the company’s most widely-used implantable cardioverter...
Internists and geriatricians have insufficient understanding of implantable cardioverter defibrillator use
Physicians in internal medicine and geriatrics and even physicians working in cardiology departments lack basic knowledge of treatment with implantable cardioverter defibrillators (ICDs) and...
US FDA approves Biotronik’s Intica CRT-DX therapy implantable cardioverter defibrillator systems
The US Food and Drug Administration (FDA) has approved Biotronik’s Intica DX and Intica cardiac resynchronisation therapy (CRT)-DX implantable cardioverter defibrillator (ICD) systems, which...
HRS 2017: Long detection in single chamber ICDs cuts mortality and...
Unnecessary shocks and all-cause mortality are reduced in patients treated with a single chamber implantable cardioverter defibrillator (ICD) programmed with a long detection window.
Lead...
High survival rates for elderly patients with implantable defibrillators
Of patients over age 65 who received an implantable cardioverter-defibrillator (ICD) after surviving sudden cardiac arrest or a near-fatal arrhythmia, almost 80% survived two...
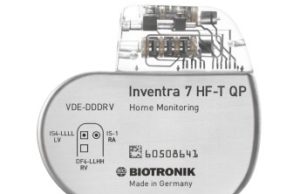
Biotronik launches Inventra ICD for heart failure patients with higher defibrillation...
Biotronik has announced the US launch of Inventra HF-T, an implantable cardioverter defibrillator (ICD) that delivers “ultra-high” energy with 42 joules (J) on the...
ESC 2016: Remote monitoring for heart failure may not improve clinical...
Two randomised clinical trials presented at a Hot Line session of the European Society of Cardiology Congress (ESC; 27‒31 August, Rome, Italy) have shown...
ESC 2016: Rethink of ICD implantation in non-ischaemic heart failure may...
Implantable cardioverter-defibrillators (ICDs) do not improve overall long-term survival compared to medical treatment in patients with non-ischaemic systolic heart failure, according to results of...
First risk score developed for preventing sudden cardiac death in the...
Researchers in the USA have identified the first generalisable risk score for sudden cardiac death. This prediction model, the researchers argue, provides well-calibrated, absolute...