 Abbott has announced the first global procedures have been conducted using the company’s new Volt pulsed field ablation (PFA) system to treat patients with arrhythmias such as atrial fibrillation (AF).
Abbott has announced the first global procedures have been conducted using the company’s new Volt pulsed field ablation (PFA) system to treat patients with arrhythmias such as atrial fibrillation (AF).
Over 30 patients were recently treated in Australia as part of Abbott’s Volt CE mark study, a premarket, multicentre clinical study designed to evaluate the safety and effectiveness of Abbott’s Volt PFA System. In addition to upcoming procedures in markets across Asia Pacific and Europe, Abbott anticipates approval for its US clinical trial (IDE) for the Volt PFA System in the first half of this year.
“We have long known that pulsed field ablation could open up an entirely new frontier in how we treat people battling the most complex cardiac arrhythmias. But like any innovation, early solutions have not been able to fully capitalize on those potential benefits,” said Prash Sanders (University of Adelaide, Adelaide, Australia), who conducted the first procedures with the Volt PFA system. “Abbott has designed a novel PFA solution that, when combined with its EnSite X cardiac mapping system, can address hard-to-treat irregular heartbeats with a level of accuracy and precision that’s never before been possible.”
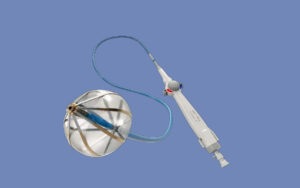
Abbott’s Volt PFA system pairs its balloon-in-basket catheter with Abbott’s EnSite X EP heart mapping system.
“Daily life for the millions of people with AF can be difficult as symptoms often include palpitations, shortness of breath, dizziness and chest pain, making it critical that physicians treat the issue as soon as possible,” said Christopher Piorkowski, chief medical officer of Abbott’s electrophysiology business. “With AF cases expected to rise continuously, Abbott’s Volt PFA system meets a growing demand for a more innovative solution that reduces the patient procedure time and overall hospital stay, getting them back to living a fuller, longer life.”












