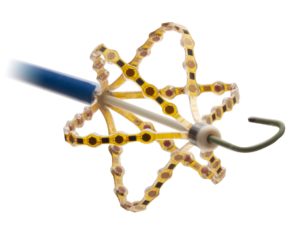
Acutus Medical has announced CE mark approval for a broad suite of electrophysiology (EP) products including the AcQCross family of universal transseptal crossing devices, the next generation AcQGuide MAX and VUE large bore delivery sheaths and the next generation AcQMap mapping catheter.
These products are designed to streamline procedural workflow in all left heart procedures and further improve ease-of-use of Acutus’ proprietary non-contact mapping technology, the company said in a press release. The devices are intended to allow electrophysiologists to quickly and accurately map the most complex atrial arrhythmias in minutes.
“Our R&D investments are translating into meaningful clinical innovations, as we build a comprehensive portfolio of therapy management solutions that make procedures safer, simpler and more effective,” said Vince Burgess, president and CEO of Acutus Medical. “We remain steadfast in our commitment to improve the treatment of complex atrial arrhythmias, and the European launch of our next generation access and diagnostic technologies is another step in that direction.”









