Append Medical, developer of the Appligator, a novel left atrial appendage (LAA) closure device to minimise stroke risk in atrial fibrillation (AF) patients, and Sheba Medical Center (Ramat Gan, Israel), have announced the clinical proof-of-concept data evaluating the safety of LAA closure through Append’s patented tissue manipulation procedure.
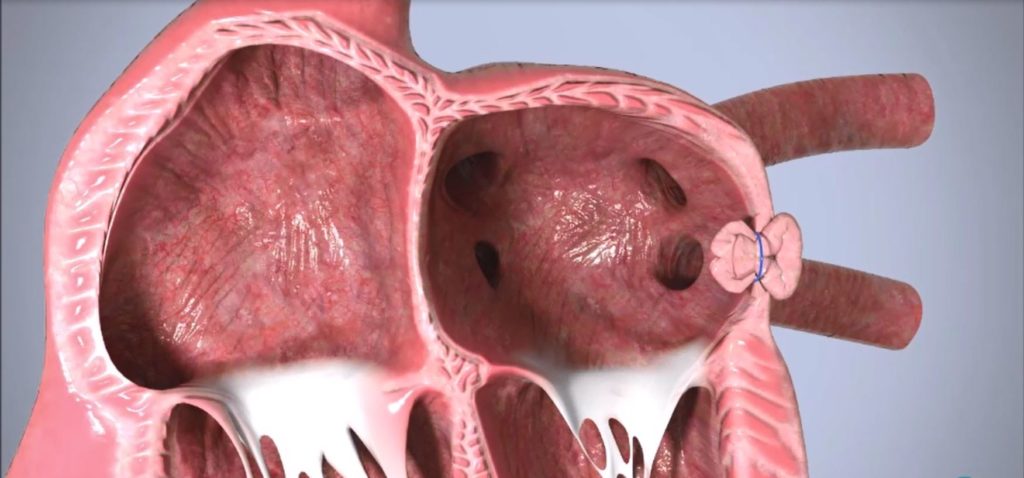
The procedure serves as a feasibility study for Append’s Appligator minimally invasive LAA closure device, which utilises natural tissue manipulation to achieve complete LAA closure through invagination of the LAA into the left atrium, leaving only a suture to seal the area.
Leonid Sternik, co-inventor of the Appligator device and director of the Department of Cardiac Surgery at Sheba Medical Center, performed the procedures in open heart surgery on two patients, both of whom suffered from AF, in clinical safety trials. In follow up after three years and one year respectively, each patient is healthy and the invaginated LAA has been successfully absorbed into the surrounding tissue of the left atrium.
“These clinical safety trials showcase the efficacy of tissue manipulation for LAA closure in atrial fibrillation patients. The LAA is a complex structure, which may lead to complications when using implanted devices for sealing. Using the LAA tissue itself for closure with no implanted device left behind can reduce risks of device-related thromboembolism and device embolisation,” said Sternik. “We believe this approach can significantly simplify LAA closure, making it safer and more effective for patients with AF-related stroke risk.”
The Append Medical Appligator device is designed to reduce stroke risk in AF patients by completely closing the LAA to prevent blood clot leakage with a minimally invasive transseptal intervention. Its design is intended to minimise device-related thromboembolism risk by leaving only a suture – and no metal implant – at the closure site.
“By developing a safer, more effective LAA closure procedure, Append is accelerating the shift from blood thinners to transseptal interventions for stroke risk reduction in AF patients, proving its value as a differentiated solution in the LAA closure market,” said Zachi Berger, founder and CEO of Append Medical.












