Jamie Bell
Regular exercise reduces AF recurrence by nearly 50% after catheter ablation,...
New research led by scientists from the University of Colorado (CU) Anschutz (Aurora, USA) suggests that staying physically active after heart rhythm treatment may...
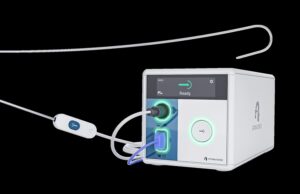
UK hospitals pilot Abbott’s Volt pulsed field ablation system in AF...
Abbott announced recently that its Volt pulsed field ablation (PFA) system is being used in procedures across the UK to treat people with atrial fibrillation...
Biotronik launches “world’s first” CRT-D devices approved for conduction system pacing
Biotronik has announced the launch of the new Acticor Sky and Rivacor Sky device family featuring “the world's first” CE mark-approved left bundle branch...
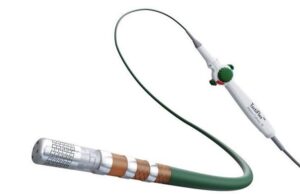
Pulse Biosciences presents latest data from first-in-human study evaluating nPulse cardiac...
Pulse Biosciences has announced late-breaking clinical data from the nPulse cardiac catheter first-in-human feasibility study, demonstrating the successful treatment of atrial fibrillation (AF) across...
Atraverse showcases findings indicating mitigated left atrial injury and reduced RF...
Atraverse Medical has announced the presentation of new clinical and preclinical data at the 2026 AF symposium (5–7 February, Boston, USA), further validating the...
Abbott announces late-breaking data from global VOLT-AF and FOCALFLEX studies
Abbott has announced new clinical data from two late-breaking presentations at the 2026 AF symposium (5–7 February, Boston, USA) that demonstrate the safety and efficacy...
Argá Medtech reports 94% lesion durability and “strong safety profile” with...
Argá Medtech has announced positive clinical results from the BURST-AF first-in-human trial, demonstrating the safety, effectiveness and durability of its Coherent sine-burst electroporation (CSE)...
Field Medical presents late-breaking Field PULSE trial data at 2026 AF...
Field Medical has announced the presentation of late-breaking clinical trial data from the first-in-human Field PULSE study—which is evaluating the company’s FieldForce ablation system...
J&J presents early outcomes from OMNY-AF pilot study alongside new Varipulse...
Johnson & Johnson (J&J) has announced 12-month pilot-phase data from the OMNY-AF study evaluating its investigational Omnypulse platform for the treatment of symptomatic paroxysmal...
Volta Medical’s RESTART trial provides “promising findings” on AI solutions in...
Volta Medical has announced results from the RESTART trial, published in the journal Heart Rhythm, that demonstrate positive outcomes for patients with recurrent atrial fibrillation...
AF-B-STEP project seeks to improve how AF is detected, quantified and...
An international research consortium has announced the launch of AF-B-STEP, a four-year research project designed to improve how atrial fibrillation (AF) is detected, quantified...
Corify publishes research evaluating new whole-heart mapping technology
Corify Care has announced a new publication on its proprietary Global Volumetric Mapping technology in Nature Communications Medicine. The company says this technology represents the...
Cathvision shares clinical progress with PFAnalyzer in pulsed field ablation
Cathvision has announced “significant clinical and scientific progress” in pulsed field ablation (PFA) with its PFAnalyzer software module, which the company says reinforces its...
Biotronik announces first human implantations of LivIQ leadless pacemaker
Biotronik has today announced the successful first-in-human implantations of its LivIQ leadless pacemaker system as part of the BIO|CONCEPT.LivIQ study—a premarket clinical investigation designed...
Medtronic’s Sphere-360 ablation catheter gains CE mark alongside first uses in...
Medtronic has today announced two major milestones relating to the Affera Sphere-360 catheter—the receipt of a CE mark in Europe and completion of the first cases...
Abbott’s TactiFlex Duo ablation catheter receives CE mark for atrial fibrillation...
Abbott announced this week that it has received a CE mark in Europe for the sensor-enabled TactiFlex Duo ablation catheter to treat patients with...
Cardiac Rhythm News’s top stories of 2025
1. Use of antidepressant medication linked to substantial increase in sudden cardiac death risk
The use of antidepressant (AD) medications has been linked to a...
Medtronic announces US commercial launch of OmniaSecure defibrillation lead
Medtronic has commercially launched the OmniaSecure defibrillation lead in the USA this week, with the first cases being performed at hospitals across the country.
OmniaSecure...
AliveCor announces US FDA clearance of new cardiac determinations for Kardia...
AliveCor has announced it has received US Food and Drug Administration (FDA) clearance for the next generation of KAI 12L—the artificial intelligence (AI) technology...
Abbott and AtaCor collaborate to advance extravascular ICD technology
Abbott has announced a collaboration with AtaCor Medical to advance a next-generation investigational extravascular implantable cardioverter defibrillator (EV-ICD) system designed to deliver defibrillation therapy...
Accurkardia launches AccurECG 2.0 following US FDA 510(k) clearance
Accurkardia has announced US Food and Drug Administration (FDA) 510(k) clearance and the commercial launch of its AccurECG analysis system (v2.0). AccurECG 2.0 builds...
Luma Vision completes Verafeye 4D-guided persistent AF ablations demonstrating advancement in...
Luma Vision has announced the successful completion of 15 Verafeye-guided persistent atrial fibrillation (AF) ablation procedures conducted as part of a clinical study in...
Stereotaxis secures US FDA approval for Magic ablation catheter
Stereotaxis has today announced that it has obtained US Food and Drug Administration (FDA) approval for the Magic magnetic interventional ablation catheter—a robotically navigated...
Abbott gains US FDA approval to treat AF patients with Volt...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the company's Volt pulsed field ablation (PFA) system to treat patients...
Cathvision releases ECGenius system version 3.5 and appoints new independent board...
Cathvision has announced the appointment of Eric Thepaut as the company’s independent chairman of the board, and the commercial release of version 3.5 of...
HeartBeam receives US FDA clearance for at-home arrhythmia assessment with cable-free...
HeartBeam has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the company’s “groundbreaking” 12-lead electrocardiogram (ECG) synthesis software...
Boston Scientific obtains CE mark to treat right atrial flutter with...
Boston Scientific Corporation has received a CE mark for the Farapoint pulsed field ablation (PFA) catheter, which is intended for use in the treatment...
Medtronic shares latest Enlighten and LEADR study results at APHRS 2025
Medtronic has announced the recent presentation of results from two late-breaking studies at the 2025 Asia Pacific Heart Rhythm Society (APHRS) Scientific Sessions (12–15...
Ablation reduces stroke risk in AF and may remove need for...
Catheter ablation—a minimally invasive procedure to correct irregular heart rhythms—may reduce the risk of stroke to such an extent that some patients can discontinue...
First patient enrolled in RESET-AF 3D study of Biotronik’s novel pulsed field ablation system
Biotronik has announced the enrolment of the first patient in the RESET-AF 3D study, a multicentre clinical study evaluating the Elepulse pulsed field ablation...
LAA closure fails to achieve non-inferiority versus standard care in AF...
For older people with irregular heart rhythms who are at high risk of stroke and bleeding, standard care—including the timely use of anticoagulant blood...
Diabetes drug reduces irregular heartbeat events in overweight adults with AF
People with atrial fibrillation (AF) and obesity may have fewer episodes of AF after catheter ablation treatment if they take the diabetes medication metformin...
One month of post-stent anticoagulation found to be as effective as...
New research has found that a simplified regimen of clot-preventing medication following stent placement in adults with atrial fibrillation (AF) was just as safe...
Volta announces US launch of AI-powered AF-Xplorer II system for complex...
Volta Medical has announced the US launch of the AF-Xplorer II system, the company's next-generation artificial intelligence (AI) solution designed to simplify real-time assessments...
J&J to showcase PFA advancements via multiple clinical and real-world studies...
Johnson & Johnson (J&J) MedTech has announced that new clinical and real-world data from its integrated-by-design Varipulse platform in pulsed field ablation (PFA) procedures for...
AtriCure announces treatment of first patient in BoxX-NoAF clinical trial
AtriCure has announced that the first patient was recently enrolled and treated in the BoxX-NoAF clinical trial, which is assessing the safety and effectiveness...
YorLabs gains US FDA 510(k) for “first-of-its-kind” intracardiac imaging system
YorLabs, a medical technology company developing next-generation intracardiac imaging solutions for electrophysiology (EP) and interventional cardiology (IC) procedures, has announced that the US Food...
Stereotaxis and CardioFocus collaborate to advance robotic PFA for cardiac arrhythmias
Stereotaxis and CardioFocus have announced that they have entered into a collaboration agreement to advance robotic pulsed field ablation (PFA) technology towards commercialisation.
This collaboration is set...
Biotronik announces first procedure involving CRT-D implant developed for LBBA pacing
Biotronik has today announced the successful first-in-human implantation of a device from its new Acticor/Rivacor Sky implantable cardioverter defibrillator (ICD)/cardiac resynchronisation therapy-defibrillator (CRT-D) portfolio...
Erasmus Medical Center performs first procedures with Genesis robotic system
Stereotaxis has announced the successful first procedures by physicians at Erasmus University Medical Center in Rotterdam, The Netherlands, using the company’s Genesis robotic magnetic...
Boston Scientific gains expanded CE-mark indication for Ingevity+ pacing leads to...
Boston Scientific has received CE-mark approval to expand the indication for its current-generation Ingevity+ pacing leads—thin wires that are implanted in the heart and...
Argá Medtech announces first enrolments in COHERENT-AF IDE clinical trial
Argá Medtech has announced initial enrolments in the COHERENT-AF pivotal clinical trial. This US Food and Drug Administration (FDA) investigational device exemption (IDE), prospective,...
Medtronic initiates global study examining cardiac pacing in HFpEF patients
Medtronic has announced the initiation of a pivotal study evaluating the use of elevated and personalised cardiac pacing rates for the treatment of patients...
AccurKardia announces clinical pilot study of ECG-based, AI-powered hyperkalaemia detection software
AccurKardia has announced the initiation of a multicentre clinical pilot study of AK+ Guard—a US Food and Drug Administration (FDA) Breakthrough Device designated, artificial...
No benefit seen when using biomarker-based risk scores to personalise AF...
An individually tailored, multidimensional risk-based treatment strategy was not associated with improvements in clinical outcomes compared with usual guideline-based care in patients with atrial...
Biotronik launches Solia CSP S with aim of simplifying conduction system...
Biotronik has announced the market release of Solia CSP S—the latest innovation in its growing portfolio of conduction system pacing (CSP) solutions. Solia CSP S is the “first...
HeartBeam’s deep learning algorithms demonstrate high rates of accuracy in detecting...
HeartBeam has announced new study data demonstrating no significant differences in the detection of atrial fibrillation (AF), atrial flutter and sinus rhythm when the...
Boston Scientific announces late-breaking data on Farapulse and Watchman FLX technologies...
At the 2025 European Society of Cardiology (ESC) congress (29 August–1 September, Madrid, Spain), Boston Scientific shared two late-breaking presentations pertaining to its Farapulse...
Study finds comparable outcomes between reconditioned and new pacemakers
Procedure-related infection rates are similar between reconditioned and new pacemakers, potentially offering a "new hope" to patients in low- and middle-income countries (LMICs), according...
StopAfib.org marks National AF Awareness Month by launching ‘Get in Rhythm....
In recognition of National Atrial Fibrillation (AF) Awareness Month in the USA (September), StopAfib.org has launched the 2025 ‘Get in Rhythm. Stay in Rhythm.’...
Digital twin technology helps reduce atrial arrhythmia recurrence after catheter ablation...
Adding digital twin-guided ablation to a standard ablation technique can improve outcomes in patients with persistent atrial fibrillation (AF), according to late-breaking research presented...
Kardium receives US FDA approval for Globe pulsed field ablation system
Kardium has announced that it has received premarket approval (PMA) for the Globe pulsed field ablation (PFA) system, and 510(k) clearance for both the...
Procedural success with PFA “not superior” to radiofrequency ablation in paroxysmal...
Pulsed field ablation (PFA) did not demonstrate superior efficacy to radiofrequency (RF) ablation in patients with drug-resistant, paroxysmal atrial fibrillation (AF) within the BEAT-PAROX-AF...
Stereotaxis announces first US commercial mapping procedures with Magic Sweep catheter
Stereotaxis has announced the successful completion of the world’s first procedures using Magic Sweep—the “first and only” robotically navigated, high-density electrophysiology (EP) mapping catheter....
Multiple datasets suggest positive safety and performance with OmniaSecure defibrillation lead
Researchers recently presented three new datasets reinforcing the safety and performance of Medtronic’s OmniaSecure defibrillation lead at the 2025 European Society of Cardiology (ESC)...
ICDs fail to reduce mortality in selected heart attack patients with...
Prophylactic implantable cardioverter defibrillator (ICD) therapy does not appear to reduce mortality in patients with a prior myocardial infarction (MI), persistent moderate left ventricular...
J&J unveils real-world results from VARIPURE substudy at ESC 2025
Johnson & Johnson (J&J) MedTech has today announced acute safety and effectiveness results from the VARIPURE substudy of the SECURE study evaluating the company’s Varipulse...
Remote AF screening using an ECG patch demonstrates modest benefits
A mail-based atrial fibrillation (AF) screening programme with electrocardiogram (ECG) patch monitoring led to a modest long-term increase in AF diagnosis and anticoagulation exposure...
New data indicate lifelong anticoagulation “might not be necessary” in all...
Discontinuing oral anticoagulation (OAC) therapy may result in a lower risk of a composite of stroke, systemic embolism or major bleeding versus continuing OAC...
Increasing potassium levels improves outcomes in patients at high risk of...
Targeting high-normal potassium levels can reduce the risk of arrhythmias, hospitalisation for heart failure or arrhythmia, and death, compared with no intervention, according to...
Conformal closes US$32 million Series D extension to advance next-generation LAAO...
Conformal Medical has announced the successful closing of its Series D extension financing, with a new partner joining inside investors to raise a total...
Pulsecare secures Chinese NMPA approval for nanosecond PFA system
Pulsecare Medical has announced that its innovative NxPFA nanosecond pulsed field ablation (ns-PFA) system has received marketing approval from China's National Medical Products Administration (NMPA).
As the...
Omron’s smart devices help detect early heart failure risks in more...
Omron Healthcare announced recently that it has successfully concluded a three-month study demonstrating how remotely monitored vital signs can facilitate early medical intervention in...
Philips announces collaboration with Epic to enhance ambulatory cardiac monitoring
Royal Philips has announced a collaboration through which the company will integrate its suite of cardiac ambulatory monitoring and diagnostics services with Aura, the specialty...
Cardiosense receives US FDA 510(k) clearance for CardioTag device
Cardiosense has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the CardioTag device—claimed by the company to be...
Stereotaxis receives US FDA clearance for Magic Sweep catheter
Stereotaxis has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Magic Sweep catheter—described by the company as...
Biotronik and CardioFocus partner to expand pulsed field ablation access in...
Biotronik has announced a strategic distribution agreement with CardioFocus to advance ablation treatment for cardiac arrhythmias, with Biotronik serving as the exclusive distributor of...
South Korea’s MFDS clears clinical trial of HyperQure renal denervation system...
DeepQure has announced that South Korea's Ministry of Food and Drug Safety (MFDS) has approved a clinical trial of the company’s HyperQure renal denervation (RDN) system—a...
Myocardial fibrosis may be linked to dangerous heart arrhythmias in male...
Scar tissue in the heart—myocardial fibrosis—may be associated with abnormal heart rhythms among healthy, long-time male endurance athletes aged 50 years or older, potentially...
US study finds association between post-ablation visual auras and signs of...
Researchers from the University of California San Francisco (UCSF) in San Francisco, USA recently set out to better understand the relationship between transseptal puncture...
AtriCure completes enrolment in trial assessing stroke prevention with AtriClip device
AtriCure has announced the completion of enrolment in the LeAAPS clinical trial—a prospective, randomised, blinded, superiority investigational device exemption (IDE) trial evaluating the AtriClip...
Philips launches ECG AI marketplace to boost availability of cardiac diagnostic...
Royal Philips has announced the launch of the Philips electrocardiogram (ECG) artificial intelligence (AI) marketplace—a platform that gives cardiac care teams access to multiple vendor...
Boston Scientific receives US FDA approval for expanded labelling of Farapulse...
Boston Scientific Corporation has received US Food and Drug Administration (FDA) approval to expand the instructions for use (IFU) labelling for the Farapulse pulsed...
Stereotaxis announces publication of first clinical results with MAGiC catheter
Stereotaxis has announced the publication in the Journal of Interventional Cardiac Electrophysiology of initial clinical results from a first-in-human study utilising the MAGiC catheter—the...
Field Medical, Kardium announce funding rounds to boost PFA systems
Field Medical and Kardium—two companies developing pulsed field ablation (PFA) systems for the treatment of heart rhythm disorders—have secured funding rounds worth US$35 million...
Cathvision announces latest software release and new board appointment
Cathvision has announced the market release of ECGenius system version 3.4, introducing multiple software enhancements aimed at improving procedural workflow and physician usability across...
J&J launches Varipulse platform across Asia-Pacific region
Johnson & Johnson (J&J) MedTech has announced the launch of the Varipulse platform—a system used to perform catheter ablation procedures for the treatment of...
UK NICE recommends pulsed field ablation as AF treatment option
The UK’s National Institute for Health and Care Excellence (NICE) has today published new guidance recommending the use of pulsed field ablation (PFA) in...
Atrial fibrillation linked to reduced first-pass effect in MeVO stroke thrombectomy...
The presence of atrial fibrillation (AF) may reduce the likelihood of mechanical thrombectomy being successful at the first attempt in patients with acute ischaemic...
EAN 2025: heart rate variability during sleep may reveal early signs...
New research presented at the 2025 European Academy of Neurology (EAN) congress (21–24 June, Helsinki, Finland) has uncovered a notable link between nighttime heart...
Volta expands AF-Xplorer system’s US labelling to include TAILORED-AF trial data
Volta Medical has announced a “significant update” to the US labelling of its flagship system, Volta AF-Xplorer. Labelling will now include “groundbreaking” clinical data...
STEEER-AF: online education can enhance clinical guideline use by more than...
The international STEEER-AF study has shown that targeted online education on atrial fibrillation (AF) for health professionals can improve guideline-adherent care. This cluster-randomised controlled trial,...
Atraverse closes US$29 million to boost commercialisation of Hotwire transseptal access...
Atraverse Medical has announced the close of US$29.4 million in follow-on financing to accelerate growth, building upon the US$12.5 million in prior seed investment...
PaceMate targets commercial expansion after appointing JR Finkelmeier as new CEO
Cardiac monitoring company PaceMate has announced the appointment of JR Finkelmeier as its new chief executive officer (CEO).
Finkelmeier joined PaceMate as chief commercial officer...
J&J MedTech announces US launch of ICE catheter for use in...
Johnson & Johnson (J&J) MedTech has announced the US launch of the Soundstar Crystal ultrasound catheter for intracardiac echocardiography (ICE) imaging in cardiac ablation procedures.
The...
Omron introduces AI-powered AF detection into home blood pressure monitoring technology
Omron Healthcare has introduced home blood pressure monitors with built-in, artificial intelligence (AI)-powered detection of atrial fibrillation (AF). In what the company describes as...
Philips introduces VeriSight Pro 3D ICE catheter in Europe
Royal Philips has today announced the introduction of its VeriSight Pro 3D intracardiac echocardiography (ICE) catheter in Europe. Building on its success in the USA,...
Kenyan study finds “striking” heart failure detection results with AI-powered ECG...
An artificial intelligence (AI)-enabled, electrocardiogram (ECG)-based algorithm has performed well in the early detection of heart failure among healthcare-seeking individuals in Kenya, according to...
Purpose-built systems hold “extremely important” advantages as PFA treatments continue upward...
This advertorial is sponsored by Boston Scientific.
Given the ever-increasing wealth of clinical data and experiences involving pulsed field ablation (PFA) for the treatment of...
HeartBeam’s synthesised 12-lead ECG meets clinical endpoints in VALID-ECG study
HeartBeam has announced that its “groundbreaking” synthesised 12-lead electrocardiogram (ECG) successfully met the clinical endpoints in the VALID-ECG pivotal study. Thomas Deering (Piedmont Heart...
Multicentre study suggests GLP-1 receptor agonists may reduce AF-related events in...
The TRANSFORM-AF study has found that patients with atrial fibrillation (AF) and obesity who were treated with a glucagon-like peptide-1 receptor agonist (GLP-1 RA)...
Circa Scientific announces US FDA clearance of PeriCross epicardial access kit
Circa Scientific has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the PeriCross epicardial access kit (formerly Rook),...
Implicity’s EVIDENCE-RM study demonstrates “marked improvement” in ICD patient outcomes
Implicity has revealed new findings from its EVIDENCE-RM study, which—by leveraging extensive data from the French National Health Data System (SNDS)—has demonstrated clear clinical...
Research unveiled at HRS 2025 highlights AI’s ability to enhance cardiac...
New research unveiled at the 2025 Heart Rhythm Society (HRS) annual meeting (24–27 April, San Diego, USA) has underscored the critical role of artificial...
Abbott, Medtronic announce positive data on respective PFA systems at HRS...
Among a great number of presentations relating to pulsed field ablation (PFA) treatments for atrial fibrillation (AF) at this year’s Heart Rhythm Society (HRS)...
Kardium announces “impressive” one-year PULSAR IDE study results with Globe PF system
Kardium has announced late-breaking, one-year clinical trial results with its innovative pulsed field ablation (PFA) device—the Globe PF system.
Pivotal findings from the PULSAR...
HRS and ACC release scientific statement on safety of same-day discharge...
The Heart Rhythm Society (HRS) and the American College of Cardiology (ACC) have released a joint scientific statement on new guiding principles for same-day...
Field Medical announces promising first-in-human data on treating scar-related VT using...
Field Medical has announced that its FieldForce ablation system was recently featured in a scientific presentation at the 2025 Heart Rhythm Society (HRS) annual...
Abbott highlights new Aveir data and initiates CSP technology trial
Abbott has announced late-breaking data from the AVEIR conduction system pacing (CSP) acute clinical feasibility study, demonstrating the safety and performance of the company’s investigational...
Medtronic receives US FDA approval for OmniaSecure lead and announces investigational...
Medtronic has received US Food and Drug Administration (FDA) approval of the OmniaSecure defibrillation lead for placement within the right ventricle. The lead—based on...
Atraverse presents clinical data on Hotwire transseptal access system at HRS...
Atraverse Medical has announced that the latest clinical evidence on the efficacy and safety of its Hotwire left heart access system were presented at...
Second phase of Boston’s ADVANTAGE AF study meets primary safety and...
Boston Scientific has announced positive 12-month primary endpoint results from the second phase of the ADVANTAGE AF clinical trial evaluating the use of the...
J&J MedTech presents new Omny-IRE and VARIPURE study data at HRS...
Johnson & Johnson (J&J) MedTech has announced positive initial three-month results from the Omny-IRE study evaluating the investigational Omnypulse platform in patients with paroxysmal...
SoloPace announces US FDA clearance and first-in-human use of TAVI temporary...
SoloPace Incorporated has announced both US Food and Drug Administration (FDA) clearance and first-in-human use of its SoloPace control system for temporary pacing in...
Stereotaxis features first-ever live demonstration of GenesisX robotic system at HRS...
Stereotaxis announced recently that it hosted a demonstration of the GenesisX robotic system at this year’s Heart Rhythm Society (HRS) meeting (24–27 April, San...
US FDA approves “world’s first and only” leadless LVEP device for...
EBR Systems has received US Food and Drug Administration (FDA) approval of the WiSE system—marking what the company describes as a significant leap forward...
US Heart Rhythm Society announces new framework to establish AF centres...
The US Heart Rhythm Society (HRS) has released a framework outlining criteria for establishing an atrial fibrillation (AF) centre of excellence (CoE) and key...
Field Medical closes US$40 million Series A financing to advance PFA...
Field Medical has announced the successful closing of US$40 million in Series A financing. The round includes US$20 million in new capital and the conversion of US$20 million in seed-round...
LBBP proves superior to biventricular pacing in reducing long-term mortality and...
A left bundle branch pacing (LBBP) approach has been shown to be superior to biventricular pacing with regard to reducing long-term all-cause mortality and...
Luma Vision receives US FDA clearance for Verafeye 2D/4D cardiac visualisation...
Luma Vision has announced the US Food and Drug Administration (FDA) clearance of its Verafeye visualisation platform.
As per a company press release, this...
Volta Medical appoints Bill Hoffman as executive chairman of its board...
Volta Medical—a company that develops artificial intelligence (AI) solutions to assist electrophysiologists—has announced the appointment of Bill Hoffman as executive chairman of its board of directors.
A...
SINGLE SHOT CHAMPION data leave “no doubt” that PFA is non-inferior...
At this year’s European Heart Rhythm Association (EHRA) congress (30 March–1 April 2025, Vienna, Austria), a late-breaking presentation of data from the SINGLE SHOT...
Use of antidepressant medication linked to substantial increase in sudden cardiac...
The use of antidepressant (AD) medications has been linked to a substantial increase in the risk of sudden cardiac death (SCD), as per research...
European registry elucidates safety outcomes of ventricular arrhythmia treatment with Affera...
A novel cardiac ablation system may hold the potential to improve upon the currently available treatments for ventricular arrhythmia (VA) patients, having demonstrated positive...
Post-TAVI pacemaker implantation linked to long-term increase in mortality rates
Pacemaker implantation within 30 days after a transcatheter aortic valve implantation (TAVI) procedure has been found to correlate with significantly higher rates of all-cause...
Medtronic announces first patient enrolment in trial assessing VT treatment with...
Medtronic recently announced that the first patient has been enrolled in a US Food and Drug Administration (FDA) early feasibility study evaluating the Affera...
Biological heart age measured via AI analyses of ECG data could...
A new study presented by researchers at the 2025 European Heart Rhythm Association (EHRA) congress (30 March–1 April, Vienna, Austria) demonstrates how, using artificial...
ESC releases “groundbreaking” consensus statement on conduction system pacing
The European Society of Cardiology (ESC) has released what it describes as a “groundbreaking” consensus statement on conduction system pacing (CSP), marking a “significant...
J&J’s dual-energy ablation catheter deemed safe and effective in first-in-human SmartfIRE...
Johnson & Johnson (J&J) MedTech has today announced 12-month results from the SmartfIRE study, delivered as a late-breaking presentation at the ongoing European Heart...
Farapulse PFA system demonstrates superior efficacy to cryoablation catheter in paroxysmal...
New findings from the SINGLE SHOT CHAMPION trial, presented at the ongoing European Heart Rhythm Association (EHRA) congress (30 March–1 April 2025, Vienna, Austria)...
PROFID EHRA: A landmark trial attempting to redefine prevention of sudden...
In a Cardiac Rhythm News guest article, chief investigators Nikolaos Dagres and Gerhard Hindricks (both Berlin, Germany) provide a window into the PROFID EHRA...
Abbott receives CE-mark approval for Volt pulsed field ablation system
Abbott announced yesterday that it has received CE-mark approval in Europe for the Volt pulsed field ablation (PFA) system to treat patients with atrial...
Arrhythmia management experts to examine device therapy, sudden cardiac death, AI...
This year’s congress of the European Heart Rhythm Association (EHRA; 30 March–1 April, Vienna, Austria), a branch of the European Society of Cardiology (ESC),...
AtaCor Medical enrols first patients in ASCEND EV clinical study
AtaCor Medical has announced that it has initiated its ASCEND EV study—a prospective, non-randomised international study aiming to evaluate the safety and performance of...
Robot detects side-effects faster than physicians during cardiac arrhythmia treatment
A software robot has proved to be faster than doctors at detecting side-effects during a drug treatment for cardiac arrhythmia, while also cutting unnecessarily...
MedLumics announces publication of first-in-human results with AblaView PFA system
MedLumics has announced the publication of first-in-human results for the AblaView pulsed field ablation (PFA) system in atrial fibrillation (AF) patients, including clinical safety...
EHRA doctors mark Pulse Day by highlighting “silent epidemic” of cardiac...
With cases of cardiac arrhythmias rising rapidly, cardiologists from the European Heart Rhythm Association (EHRA) have marked Pulse Day (1 March) by urging communities...
New gene identified in search for improved malignant cardiac arrhythmia therapy
Research from Amsterdam UMC (Amsterdam, The Netherlands) and Johns Hopkins University (Baltimore, USA), published recently in the European Heart Journal, could represent an important step...
J&J to resume Varipulse cases following ‘voluntary pause’ of US external...
Following a temporary, voluntary pause of US external evaluation and all US cases with its Varipulse catheter, Johnson & Johnson (J&J) is set to...
Cathvision receives European CE mark for ECGenius system
Cathvision has announced the receipt of a CE mark for its breakthrough product, the ECGenius system. According to the company, this regulatory milestone allows...
DiVERT Stroke study uncovers sex-based disparities in post-stroke cardiac care
New findings from the DiVERT Stroke clinical study show that women spent less time in the hospital, saw fewer referrals to cardiology and were...
Boston Scientific obtains CE mark for next generation of cardiac mapping...
Boston Scientific Corporation has announced it has received CE-mark approval for its navigation-enabled Farawave Nav ablation catheter in the treatment of paroxysmal atrial fibrillation...
Volta Medical’s AI-guided cardiac ablation procedure found to improve AF treatments
Volta Medical has announced the publication in Nature Medicine of the TAILORED-AF clinical trial, which demonstrated that an artificial intelligence (AI)-guided procedure for persistent atrial...
Stereotaxis receives CE-mark approval for MAGiC ablation catheter
Stereotaxis has announced that it recently received European CE-mark approval for the MAGiC ablation catheter. A press release highlights this approval is a “significant...
Lucia and Heart Rhythm Society collaborate to enhance UpBeat.org for AF...
Lucia and the Heart Rhythm Society (HRS) have announced a strategic collaboration to leverage Lucia's cutting-edge technology to enhance UpBeat.org—the HRS’ premier online resource...
Cardiac Rhythm News’ top stories of 2024
From first-time clinical data to major industry debuts, here are Cardiac Rhythm News’ 10 most popular stories of 2024.
1. Abbott joins the pulsed field...
Factor XI inhibitor ‘lives up to promise’ of reduced bleeding events...
Researchers from Mass General Brigham (Boston, USA) have evaluated a drug that represents a new class of anticoagulants known as Factor XI inhibitors for treating patients...
Pulse Biosciences announces presentation of late-breaking data on nsPFA 360-degree cardiac...
Pulse Biosciences has announced late-breaking data from the first-in-human feasibility study of its nanosecond pulsed field ablation (nsPFA) 360-degree cardiac catheter system at the...
AliveCor announces CMS reimbursement approval for Kardia 12L ECG system
AliveCor has announced that the US Centers for Medicare & Medicaid Services (CMS) has included the company's artificial intelligence (AI)-powered electrocardiogram (ECG) technology in...
Atraverse announces clinical evidence validating Hotwire radiofrequency guidewire system
Clinical evidence supporting the efficacy, safety and efficiency of Atraverse Medical's Hotwire left-heart access system was presented recently at the 30th annual AF symposium...
New data presented on Farapulse and Watchman FLX devices at AF...
Boston Scientific announced data supporting the use of the Farapulse pulsed field ablation (PFA) system and the Watchman FLX left atrial appendage closure (LAAC) device,...
Luma completes first-in-human procedures with novel Verafeye platform
Luma Vision has announced the successful first-in-human device use and enrolment in its LUMINIZE clinical study involving the “groundbreaking” Verafeye platform. A company press release...
LINQ family of AI-supported insertable cardiac monitors accurately predicts risk thresholds...
Primary results from the DEFINE AFib clinical study have found that Medtronic's LINQ family of insertable cardiac monitors (ICMs), paired with a novel algorithm,...
Study finds AliveCor’s Kardia 12L ECG performs equivalently to standard 12-lead...
AliveCor has announced new data published in the journal Computing in Cardiology (CINC) demonstrating that its Kardia 12L electrocardiogram (ECG) system—the “world's first” artificial intelligence (AI)-powered,...
Circa announces successful first procedures with Crosswise RF transseptal access system
Circa Scientific has announced the launch of its innovative Crosswise radiofrequency (RF) transseptal access system, which is designed to provide precise puncture of the septal...
Biotronik announces first implant of next-generation conduction system pacing lead in...
Biotronik has announced the first in-human implantations in the BIO-Master.CSP study examining the use of the company’s investigational, next-generation Solia conduction system pacing (CSP)...
Peerbridge Health unveils COR-INSIGHT trial to validate AI-enabled cardiac diagnostics and...
Peerbridge Health has announced the launch of its COR-INSIGHT trial, which will validate the screening and diagnostic capabilities of its Peerbridge Cor ambulatory electrocardiography...
Abbott announces “first-in-world” leadless pacing procedures in left bundle branch area...
Abbott has announced the successful completion of the “world's first” in-human leadless left bundle branch area pacing (LBBAP) procedures using the company's investigational Aveir...
HeartBeam announces US FDA clearance for at-home, high-fidelity heart monitoring technology
HeartBeam has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance of the HeartBeam system for comprehensive arrhythmia assessment. A...
US FDA issues alert over potential need for early device replacement...
The US Food and Drug Administration (FDA) has alerted patients, caregivers and healthcare providers about the potential need for early device replacement of Boston...
FieldForce ablation system earns US FDA TAP pilot acceptance and breakthrough...
Field Medical has announced that its FieldForce ablation system has been accepted into the US Food and Drug Administration (FDA)’s Total Product Life Cycle Advisory...
CathVision unveils EMR integration and new report generator in ECGenius system...
CathVision has unveiled the latest ECGenius system update—a release that brings essential enhancements to streamline workflows and boost procedural efficiency in electrophysiology (EP) labs,...
BTL Medical announces successful first-in-human treatments with novel PFA catheter
BTL Medical has announced the successful treatment of the first six patients in a first-in-human feasibility study using its next-generation catheter system designed to...
Manipal Hospitals successfully performs eastern India’s first AI-powered wireless pacemaker insertion
Manipal Hospitals—one of the largest healthcare networks in India—has announced the successful insertion of an artificial intelligence (AI)-driven wireless injectable pacemaker.
The Aveir leadless...
Pilot study shows cryoablation safe and effective for patent foramen ovale...
New research suggests that cryoablation is a safe and effective approach to closing congenital patent foramen ovale (PFO) in patients with atrial fibrillation (AF)...
Texas Cardiac Arrhythmia Institute becomes first in USA to use FDA-approved...
Electrophysiologists at the Texas Cardiac Arrhythmia Institute (TCAI) at St David's Medical Center (Austin, USA) recently became the first in the USA to use...
AHA 2024: Three trials shed new light on AF ablation outcomes
Late-breaking results from the PROMPT-AF, CRRF-PeAF and ARREST-AF trials were presented recently at the American Heart Association (AHA) Scientific Sessions (16–18 November 2024, Chicago, USA),...
Accurate prediction of sudden cardiac death following heart attack “currently not...
New research published in the European Heart Journal has shown that, among patients who have had a heart attack, it is “extremely difficult” to accurately predict...
AHA 2024: Lifestyle and risk-factor changes improve AF symptoms—not burden—compared to...
Treatment with the Type 2 diabetes medication metformin, lifestyle changes, or a combination of both, did not improve atrial fibrillation (AF) burden or progression...
COVID-19 vaccination “generally not associated” with increased thromboembolic event risk in...
In patients with atrial fibrillation (AF) or atrial flutter, COVID-19 vaccination appears to be “generally not associated with an increased risk of thromboembolic events”,...
AHA 2024: Blood thinners fail to reduce cognitive decline in younger...
Prescribing anticoagulation medications to adults younger than 65 years of age who have atrial fibrillation (AF) but no other risk factors for stroke does not...
AHA 2024: Ablation potentially superior to medication for post-heart attack VT
Ablation may be a better first-line treatment for heart attack survivors experiencing dangerous episodes of ventricular tachycardia (VT), according to late-breaking science presented recently...
AHA 2024: Reconditioned pacemakers appear to perform as well as new...
A randomised trial comparing previously used and new pacemakers in patients with heart rhythm disorders has found reconditioned devices to be as safe and...
Two innovative ECG monitors obtain new US FDA clearances
Two innovative electrocardiogram (ECG) products have gained new US Food and Drug Administration (FDA) clearances for monitoring cardiac arrhythmias—Fourth Frontier’s Frontier X Plus (FX+)...
Defibrillation devices could remain effective while using 1,000 times less electricity
In a paper published recently in the journal Chaos, researchers from Sergio Arboleda University (Bogotá, Colombia) and the Georgia Institute of Technology (Atlanta, USA) used...
Study indicates more exercise is better—but even moderate amounts may reduce...
An extra hour of physical activity every week may lower a person’s chance of developing the most common type of arrythmia by 11%, a...
University of Kansas Health System enhances robotic heart care programme with...
Stereotaxis has announced that physicians at the University of Kansas Health System (Kansas City, USA)—a system renowned for diagnosing and treating complex heart conditions—have...
Collaboration attempts to establish research capacity for ambulatory rhythm monitoring in...
A recent study published in JACC: Clinical Electrophysiology has placed emphasis on the importance of building research capacity for ambulatory rhythm monitoring in sub-Saharan...
Pulse appoints David Kenigsberg as Electrophysiology CMO and adds Andrea Natale...
Pulse Biosciences has announced the appointment of David Kenigsberg (Florida Heart Rhythm Specialists, Fort Lauderdale, USA) as chief medical officer (CMO) of Electrophysiology. In...
J&J MedTech receives US FDA approval for Varipulse PFA platform in...
Johnson & Johnson (J&J) MedTech has announced the US Food and Drug Administration (FDA) approval of the Varipulse pulsed field ablation (PFA) platform for...
“Transformative” new data advocate early blood-thinning therapy in patients with atrial...
People with atrial fibrillation (AF) who have a stroke could benefit from receiving blood-thinning treatments—known as direct oral anticoagulants (DOACs)—at an earlier stage than...
MedLumics treats first 10 patients in AblaView clinical study and announces...
MedLumics recently announced that 10 patients have been treated in the company’s first-in-man clinical trial, which is ongoing and currently under protocol-dictated follow-up. The...
Boston Scientific announces agreement to acquire Cortex
Boston Scientific Corporation announced today that it has entered into a definitive agreement to acquire Cortex, an Ajax Health company and privately held medical...
Adagio completes first procedures in pivotal study of VT cryoablation system
Adagio Medical has announced the completion of the first procedures in the FULCRUM-VT US Food and Drug Administration (FDA) pivotal investigational device exemption (IDE)...
Abbott initiates TEAM-HF trial to improve outcomes in advanced heart failure...
Abbott has announced a new, “first-of-its-kind” clinical trial designed to improve outcomes in patients with worsening heart failure who could benefit from advanced therapy...
HRS establishes new advocacy organisation to address needs of electrophysiologists and...
The Heart Rhythm Society (HRS) board of trustees has unanimously approved the formation of Heart Rhythm Advocates—a new, shared-vision, cause-related non-profit 501(c)(4) advocacy organisation...
Boston Scientific launches next generation of cardiac mapping for Farapulse PFA...
Boston Scientific Corporation has announced that it has received US Food and Drug Administration (FDA) approval of the navigation-enabled Farawave Nav ablation catheter for...
Abbott progresses PFA clinical studies, launches new advanced cardiac mapping technology
Abbott announced today that it has achieved new, major milestones to support the company's growing suite of pulsed field ablation (PFA) solutions in electrophysiology.
These...
Adjunctive coronary sinus isolation confers no additional benefits over PVI ablation...
Adjunctive coronary sinus isolation (CSI) as part of a de-novo ablation strategy does not confer any additional benefit compared to pulmonary vein isolation (PVI)...
AF pattern at first diagnosis does not appear to influence post-ablation...
A patient’s pattern of atrial fibrillation (AF) at their initial diagnosis does not influence their rate of AF recurrence, nor their AF burden, following...
HRS releases new policy statement underscoring requirements for AF catheter ablation
The Health Policy and Regulatory Affairs Committee of the Heart Rhythm Society (HRS) has published a statement online in the Heart Rhythm Journal reaffirming the important role...
Boston Scientific receives Japanese regulatory approval for Farapulse PFA system
Boston Scientific has announced receipt of Pharmaceuticals and Medical Device Agency (PMDA) approval in Japan for the Farapulse pulsed field ablation (PFA) system. The...
New clinical data show “excellent lesion durability” with Medtronic’s PulseSelect PFA...
Medtronic has announced the presentation of clinical study results demonstrating a high rate of durable lesion formation with the PulseSelect pulsed field ablation (PFA)...
Boston’s Empower leadless pacemaker demonstrates “excellent overall clinical performance” in MODULAR...
Boston Scientific recently announced late-breaking data from the MODULAR ATP clinical trial investigating the pacing performance of the company’s Empower leadless pacemaker. The data...
Cerenovus and Biosense Webster among brands newly incorporated into Johnson &...
Johnson & Johnson (J&J) recently shared that its medical technology businesses—including Cerenovus and Biosense Webster, as well as Abiomed, DePuy Synthes, and Ethicon—will now...
Merit Medical signs agreement to purchase lead management portfolio from Cook...
Merit Medical Systems has announced that it recently signed a definitive asset purchase agreement to purchase Cook Medical’s lead management portfolio for a total...
PULSE-EU: single-shot spherical array PFA catheter achieves good clinical efficacy at...
The treatment of atrial fibrillation (AF) via a novel, ‘single-shot’ spherical array approach to pulsed field ablation (PFA) has been found to achieve durable...
Final results from Medtronic’s EV ICD pivotal trial demonstrate high ATP...
Medtronic recently shared long-term results from the global EV ICD pivotal trial, reinforcing the performance and safety of the company’s extravascular implantable cardioverter defibrillator...
Quitting smoking reduces risk of atrial fibrillation, new study shows
Quitting cigarettes can significantly lower a person’s risk of atrial fibrillation (AF) compared to those who continue to smoke, according to a study published...
Boston Scientific appoints Angelo Auricchio as chief medical officer for EMEA...
Boston Scientific has announced the appointment of Angelo Auricchio as chief medical officer (CMO) of the company’s Rhythm Management business in Europe, the Middle...
ESC 2024: Mass AF screening via ECG plus heart failure biomarker...
Mass screening for atrial fibrillation (AF) using electrocardiography (ECG) together with a heart failure biomarker does not prevent ischaemic stroke or systemic in older...
Abbott brings “world’s first” dual-chamber leadless pacemaker to the UK
Abbott has announced that, as of 6 September, it will be rolling out its Aveir dual-chamber (DR) leadless pacemaker system across the UK. In...
ESC 2024: Widely performed additional ablation procedure deemed ineffective in persistent...
In patients with persistent atrial fibrillation (AF), standard treatment with pulmonary vein isolation (PVI) ablation resulted in similar outcomes to more extensive ablation in...
ESC 2024: Randomised trial shows education for healthcare professionals can improve...
Adherence to atrial fibrillation (AF) guideline recommendations was found to be poor in clinical practice across Europe—but, a structured educational programme for healthcare professionals...
HeartKey Rhythm suite launched following US FDA clearance
B-Secur has announced the US Food and Drug Administration (FDA) clearance and launch of HeartKey Rhythm—a suite of electrocardiogram (ECG) algorithms and analytics designed...
ESC 2024: Wearable monitor increases diagnoses of common heart rhythm disorder...
Screening for atrial fibrillation (AF) using a wearable heart monitor for two weeks can identify older adults with this potentially dangerous abnormal heart rhythm,...
ESC 2024: Simplified AF ablation technique proven advantageous for heart failure...
As per late-breaking research presented recently at the 2024 European Society of Cardiology (ESC) congress (30 August–2 September, London, UK), cryoballoon (CB) ablation is...
ESC 2024: Mainstay ablation procedure for atrial fibrillation shows “substantial benefit”...
A catheter ablation procedure widely used to treat the most common heart rhythm disorder significantly reduces the burden of atrial fibrillation (AF), and results...
Job strain and “imbalance between efforts applied versus rewards received” may...
Work-related stress caused by job strain and an “imbalance between efforts applied versus rewards received” may increase the risk of developing atrial fibrillation (AF),...
Danish study results advocate general anaesthesia over conscious sedation in catheter...
A study utilising nationwide healthcare data from across Denmark has indicated that, during catheter ablation procedures, conscious sedation (CS) may be linked to a...
PCORI commits US$57 million to studies evaluating beta-blocking medications and implantable...
The US Patient-Centered Outcomes Research Institute (PCORI) has announced the approval of funding awards totalling more than US$165 million for new patient-centred, comparative clinical...
Pulse Biosciences successfully treats first patients with nano-PFA cardiac surgery system
Pulse Biosciences has announced treatment of the first two patients in a first-in-human feasibility study using its novel nanosecond pulsed field ablation (nsPFA) cardiac...
Adagio and ARYA IV combine to form new company focused on...
Adagio Medical has announced the completion of its business combination with ARYA Sciences Acquisition Corp IV—a special purpose acquisition company sponsored by an affiliate...
Recent US and EU publications underscore potential impact of Jewel defibrillator
Element Science has announced the publication of results from its Jewel investigational device exemption (IDE) study—conducted at sites across the USA—in the Journal of...
Atraverse announces first clinical use of Hotwire RF guidewire system
The Hotwire radiofrequency (RF) guidewire device from Atraverse Medical has been used for the first time in clinical practice by world-renowned cardiac electrophysiologist Devi Nair...
Trial approved for new pacemaker aiming to boost recovery in heart...
Ceryx Medical has announced the approval of a trial evaluating Cysoni-XT—a new temporary cardiac pacemaker aiming to boost recovery in heart failure.
The UK Medicines...
Viz.ai and Medtronic to collaborate on improving post-cryptogenic stroke care in...
Viz.ai has announced a strategic collaboration with Medtronic to improve the coordination of cryptogenic stroke patient care between neurology and cardiology teams.
For stroke patients...
Append Medical raises US$4.35 million in initial closing of extended series...
Append Medical has raised US$4.35 million as part of an extended series A round, which will be used to support the company's first-in-human trials of Appligator.
Investors include...
Early anticoagulation after ischaemic stroke “reasonable” and “unlikely to cause harm”
An international clinical trial presented today at the European Stroke Organisation Conference (ESOC; 24–26 May, Munich, Germany) has demonstrated that—in people with ischaemic stroke...
UK MHRA awarded £10 million to fast-track patient access to medical...
A total of £10 million has been awarded to the Medicines and Healthcare products Regulatory Agency (MHRA)—an executive agency of the UK Department of...
Brainomix technology integrated into new UK atrial fibrillation study
Brainomix has announced its involvement in a new study sponsored by the University of Liverpool (Liverpool, UK) focused on post-stroke atrial fibrillation (AF). Sites...
Continuous monitoring detects atrial fibrillation in 20% of atherosclerotic stroke survivors
In a study presented yesterday at the International Stroke Conference (ISC; 8–10 February, Dallas, USA), irregular heart rhythms were detected in roughly one in...
Atrial fibrillation does not modify treatment effect of bridging thrombolysis, study...
An international study conducted by Leonard Yeo (National University Hospital, Singapore) and colleagues has found that the presence of atrial fibrillation does not modify...
Swiss parliament votes to accept US FDA-approved medical devices
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Administration (FDA) marketing authorisation in Switzerland.
A motion...
Abbott to receive multiple CES 2023 awards for neurostimulation and pacemaker...
Abbott has been recognised by the Consumer Technology Association (CTA) with three Consumer Electronics Show (CES 2023; 5–8 January, Las Vegas, USA) Innovation Awards...
CVRx launches new version of neuromodulation device intended to treat heart...
CVRx—the developer of the “world’s first” US Food and Drug Administration (FDA)-approved neuromodulation device to treat the symptoms of heart failure—has launched its new...
Medtronic names Laura Mauri as new chief scientific, medical and regulatory...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities...
Remote blood pressure monitoring beneficial for stroke survivors in under-resourced areas
A new strategy using telehealth to monitor blood pressure at home for several months immediately after a stroke had a positive impact on patient engagement and blood...
FDA issues two final guidances for including patient perspectives in medical...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As...
US physicians “among nation’s first” to implant neurostimulator technology for advanced...
Doctors at St David's Medical Center in Austin, USA recently implanted a new neurostimulator technology in patients to help treat advanced heart failure—and are...
EHRA 2021: Subcutaneous ICDs maintain cardioversion efficacy of 98% across five-year...
Subcutaneous implantable cardioverter defibrillator (S-ICD) devices have demonstrated a cardioversion efficacy rate of 98% over a period of at least five years in the...
EHRA 2021: Efforts should be made to increase participation in atrial...
Further efforts should be made to increase participation in atrial fibrillation (AF) screening—particularly in elderly populations, where systematic screening for AF can help to...