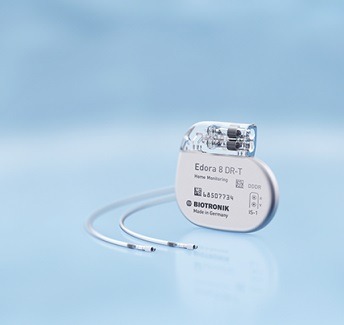
Biotronik is launching a new series of Edora single- and dual-chamber pacemakers in Japan ahead of its global launch. These devices feature both the company’s ProMRI and Biotronik Home Monitoring technology.
“The size reduction achieved with the latest Biotronik pacemakers does not compromise longevity and is more acceptable for the Japanese patient population,” comments Akihiko Yotsukura, Hokko Memorial Hospital Caress Sapporo, Hokkaido, Japan. “The devices also come equipped with the MRI AutoDetect feature. Thus these devices are not only ideal for elderly patients but all patients who may require an MRI at some point.”
According to a company release, Japan is the world’s largest MRI market with more MRI scanners per million population than any other country. Edora’s single- and dual-chamber pacemakers are currently approved for full body MRI scans at 1.5T and 3T scans with an exclusion zone.
Once activated, the MRI AutoDetect smart function is designed to automatically recognise when the patient is in an MRI environment. It then switches itself to MRI mode, and switches back to normal functionality when MRI fields are no longer detected.
According to the release, the pacemakers also have a battery life of 14 years.








