Tag: Biotronik
Biotronik announces first implant of next-generation conduction system pacing lead in...
Biotronik has announced the first in-human implantations in the BIO-Master.CSP study examining the use of the company’s investigational, next-generation Solia conduction system pacing (CSP)...
Biotronik introduces “first” complete system for LBBAP
Following the recent CE approval of the Solia S lead for left bundle branch area pacing (LBBAP), Biotronik has announced the first complete conduction...
Biotronik announces it will solely supply proprietary DX models for new...
Biotronik has announced that it will solely supply the proprietary DX models for new single-chamber implantable cardioverter-defibrillators (ICDs) implants moving forward.
The move is being...
Phillips and Biotronik to expand out-of-hospital range of cardiovascular devices
Philips, a global leader in health technology, has announced it has teamed up with Biotronik, a leading global medical device company with products and...
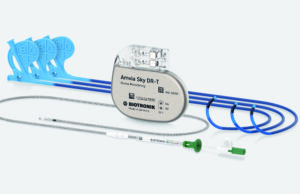
Biotronik receives CE mark for Amvia Sky and Edge devices
Biotronik announced today the latest addition to its cardiac rhythm management portfolio.
"We are excited to have received CE mark for our newest technology—the world’s...
Study results demonstrate high detection accuracy for atrial fibrillation using single-lead...
Results from the MATRIX study show that the high detection accuracy of DX single-lead implantable cardioverter defibrillator (ICD) systems for atrial fibrillation (AF) episodes...
Biotronik announce first patient enrolment for BIO-CONDUCT Solia S pacing lead...
Biotronik announce the first patient enrolment in BIO-CONDUCT, an US Food and Drug Administration (FDA) approved Investigational Device Exemption (IDE) trial examining the use...
Biotronik announces Japanese launch of injectable cardiac monitor
Biotronik has announced the market release of its injectable cardiac monitor (ICM), BioMonitor III in Japan. The device is designed to help patients with...
SENSE data show DX system is equivalent to dual-chamber ICDs and...
Biotronik has announced the publication of the SENSE trial results in the Journal of Cardiovascular Electrophysiology, proving Biotronik's DX system is equivalent to dual-chamber...
Biotronik receives FDA clearance on BioMonitor III
Biotronik has announced FDA clearance of the BioMonitor III injectable cardiac monitor (ICM). BioMonitor III is designed to document suspected arrhythmia or unexplained syncope...
BioMonitor III injectable cardiac monitor launched onto European market
After receiving the CE mark, Biotronik has launched its next-generation injectable cardiac monitor (ICM)—BioMonitor III—onto the European market. A press release reports that the...
A cardiologist’s clinical experience with BIOTRONIK’s Acticor/Rivacor* ICDs and CRT-Ds
This advertorial has been sponsored by BIOTRONIK
Dr. Victor Sanfins is a cardiologist at Hospital da Senkora da Oliveira in Guimarães, Portugal, and a specialist...
US physicians now treating patients with Biotronik’s Acticor DX devices
Biotronik recently announced the full commercial launch of the Acticor device family, including Acticor DX and CRT-DX devices. Leading electrophysiologists throughout the USA are...
Biotronik launches 3 Tesla full-body MR conditional ICD and CRT-D devices...
Biotronik today announced the European market release of the world’s smallest implantable cardioverter-defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) that are approved for...
New Australian research and development partnership aims to innovate heart failure...
Biotronik, the University of Newcastle (Callaghan, Australia) and the Hunter New England Local Health District (HNE LHD) have partnered to shape a heart failure care model. To...
BIOTRONIK opens education and innovation centre in Tokyo
BIOTRONIK have announced the opening of its Education and Innovation Centre in Ebisu, Tokyo, Japan. The centre will provide the latest educational programs and...
US FDA approves Biotronik’s Edora HF-T QP MR-condition CRT-P
The US Food and Drug Administration has approved Biotronik’s Edora HF-T QP magnetic resonance (MR)-conditional cardiac resynchronisation therapy pacemaker (CRT-P). The device—which features MRI...
US FDA approves Biotronik’s Intica CRT-DX therapy implantable cardioverter defibrillator systems
The US Food and Drug Administration (FDA) has approved Biotronik’s Intica DX and Intica cardiac resynchronisation therapy (CRT)-DX implantable cardioverter defibrillator (ICD) systems, which...
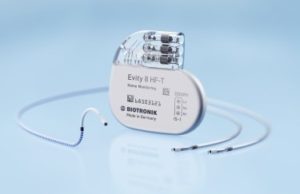
Biotronik’s Evity quadripolar CRT-P released in Japan
Biotronik’s Evity cardiac resynchronisation therapy pacemaker (CRT-P) has been launched in Japan. It is the company’s highest performing CRT-P, with a battery life of...
Biotronik receives CE mark for 3 Tesla full-body MRIs with newest...
Biotronik has received CE mark approval for 3 tesla (T) full-body scans with its latest range of magnetic resonance (MR) conditional pacemaker systems, including...
Biotronik launches Edora pacemaker series with MRI AutoDetect technology
Biotronik has announced the availability of the Edora line of devices, the company's first available pacemaker series featuring the company's magnetic resonance imaging (MRI)...
Biotronik launches first US FDA-approved CRM devices with MRI AutoDetect technology
Biotronik has announced the availability of the first US Food and Drug Administration (FDA)-approved cardiac rhythm management (CRM) devices with technology that automatically recognises...
US FDA approves Biotronik’s Sentus ProMRI quadripolar left ventricular lead
The US Food and Drug Administration (FDA) has approved Biotronik’s Sentus ProMRI, the thinnest quadripolar left ventricular lead available in the country.
Sentus ProMRI is...
Pacemakers with Closed Loop Stimulation programming help to reduce recurrence of...
Patients with refractory reflex vasovagal syncope who received a pacemaker programmed with Closed Loop Stimulation (DDD-CLS, algorithm from Biotronik) had a seven-fold reduction in...
Biotronik launches Plexa ProMRI ICD lead with helical design in Europe
Biotronik has announced the European launch of a new lead for tachycardia therapy that features a helical design for increased long-term performance through stress...
Biotronik launches Edora MR-conditional pacemakers
Biotronik has launched the Edora range of high-functioning, small-size pacemakers and cardiac resynchronisation therapy pacemakers (CRT-Ps).
The new devices are equipped with features designed to improve...
Biotronik launches a new series of Ilivia CRT devices
Biotronik has announced the launch of a new series of cardiac resynchronisation therapy (CRT) devices with several functionalities that allow physicians to offer an...
Biotronik to host 10th annual “Expert Meeting Berlin”
For the tenth year in a row, Biotronik is hosting the “Expert Meeting Berlin” (EMB). Led by Christopher Piorkowski, Heart Center Dresden, Germany, the...
Biotronik launches Edora smaller-size pacemakers
Biotronik is launching a new series of Edora single- and dual-chamber pacemakers in Japan ahead of its global launch. These devices feature both the...
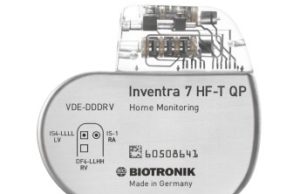
Biotronik launches Inventra ICD for heart failure patients with higher defibrillation...
Biotronik has announced the US launch of Inventra HF-T, an implantable cardioverter defibrillator (ICD) that delivers “ultra-high” energy with 42 joules (J) on the...
EHRA releases White Book 2016 EP Europace Supplement
The European Heart Rhythm Association (EHRA) and the EP Europace journal have released the supplement to its ninth annual EHRA White Book, developed in...
Biotronik Home Monitoring associated with 38% reduced all-cause mortality at one...
During a scientific talk at the 2016 European Society of Cardiology (ESC) Congress, Gerhard Hindricks, Leipzig Heart Center, Germany, presented results of a meta-study;...
First European implantation of Biotronik E3-series pacemaker performed in UK
Following recent CE mark approval for Biotronik’s E-Series pacemakers, a UK patient has become the first in Europe to be implanted with the streamlined...