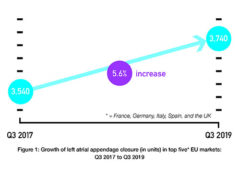
Occlutech has obtained European CE mark approval for its left atrial appendage, (LAA), occluder.
The device is a specifically designed implant for the minimally invasive closure of the LAA, a procedure that minimises the risk of strokes in patients suffering from atrial fibrillation.
The Occlutech LAA occluder consists of a flexible nitinol wire mesh with loop anchoring technology and sealing properties. The special design is intended to provide properties that enhance flexibility and adaptability resulting in a high rate of acute closure.