Tag: LAA
First patient treated with AtriClip FLEX-Mini LAA device
AtriCure has announced that the first patient has been treated with the AtriClip FLEX-Mini left atrial appendage (LAA) exclusion device, which recently received US...
AtriClip LAA exclusion system granted approval for marketing in China
AtriCure has received regulatory approval from the National Medical Products Administration (NMPA) of China to market and sell several models of its AtriClip left...
Abbott announces trial to assess new therapy option for stroke
Abbott has announced approval from the US Food and Drug Administration (FDA) for the CATALYST trial to examine its Amplatzer Amulet device compared to...
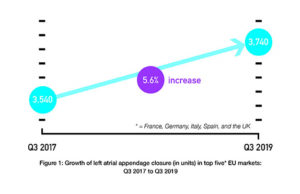
BIBA Briefings: Longest and largest follow-up data for Watchman supports its...
Data from the CAP and CAP2 registries—which contain the longest and largest follow-up of patients—add to previous evidence that left atrial appendage closure (LAAC)...
AtriCure agrees to buy LARIAT developer SentreHEART
AtriCure has entered into a definitive agreement to acquire SentreHEART, which developed the LARIAT device for left atrial appendage closure in patients with atrial...
Octreotide enables left atrial appendage closure in AF patients with GI...
Octreotide therapy has the potential to offer atrial fibrillation (AF) patients with arteriovenous malformations (AVM) related gastrointestinal (GI) bleeding another treatment option. It is...
Analysis of LAA closure devices reveals post-approval spike in safety events
A retrospective study conducting a safety assessment and comparison between two percutaneous devices commonly used for left atrial appendage (LAA) closure in the US...
Europace 2017: Thrombus formation on left atrial appendage occlusion devices strongly...
A French multicentre analysis of left atrial appendage (LAA) occlusion for stroke prevention in atrial fibrillation patients has found a 5.3% rate of thrombus...
HRS 2017: Watchman prevents stroke even in high-risk patients: One-year follow-up...
Left atrial appendage closure with the Watchman device (Boston Scientific) is safe and effective at stroke prevention, with a high implant and sealing success,...
First Texas centre uses St Jude Medical Amplatzer Amulet
The Texas Cardiac Arrhythmia Institute at St David's Medical Center, Austin, USA became the first facility in the US state of Texas to implant...
AF Symposium 2017: Use of intracardiac echocardiography is a “viable option”...
Intracardiac echocardiograpy (ICE) facilitates a high success implant rate of the Amplatzer Amulet left atrial appendage (LAA) occluder (St Jude Medical/Abbott) as well as...
Transcatheter atrial shunt benefits persist through one year in treatment of...
Twelve-month results of the REDUCE LAP HF (Reduce elevated left atrial pressure in patients with heart failure) trial have shown both continued device patency...
Charity believes NHS programme will leave hundreds of atrial fibrillation patients...
Arrhythmia Alliance, the UK-based heart rhythm charity, is calling on NHS England to reconsider a programme that it says will mean the “hundreds” of...
Commissioning through evaluation for LAAO in England
Dhiraj Gupta (Liverpool Heart and Chest Hospital, Liverpool, UK) discusses NHS England's commissioning through evaluation for left atrial appendage occlusion (LAAO). This programme is...
SentreHEART announces first clinical use of the Eclipse surgical left atrial...
SentreHEART has announced it has treated the first patients using the Eclipse surgical device for left atrial appendage (LAA) closure. Krzysztof Bartus (Jagiellonian University,...
No significant differences between Watchman and Amplatzer in first comparison
Results from the first study to compare outcomes of two left atrial appendage closure devices—Watchman (Boston Scientific) and Amplatzer (St Jude Medical)—indicate that there...
St Jude Medical launches trial of Amplatzer Amulet left atrial appendage...
The St Jude Medical Amplatzer Amulet Investigational Device Exemption (IDE) trial, which will evaluate the safety and effectiveness of the company’s Amplatzer Amulet left...
Educational supplement: LAA closure for stroke prophylaxis in atrial fibrillation
This educational supplement is only for readers in countries outside France, Japan and the USA
In this educational supplement, sponsored by Boston Scientific, Cardiac Rhythm News explores left...
CE mark granted to Occlutech left atrial appendage occluder
Occlutech has obtained European CE mark approval for its left atrial appendage, (LAA), occluder.
The device is a specifically designed implant for the minimally...