 Conformal Medical has announced the presentation of one-year data from the company’s initial left atrial appendage occlusion (LAAO) cases with its novel CLAAS system in patients under conscious sedation without general anaesthesia.
Conformal Medical has announced the presentation of one-year data from the company’s initial left atrial appendage occlusion (LAAO) cases with its novel CLAAS system in patients under conscious sedation without general anaesthesia.
Vivek Reddy, (Mount Sinai Hospital, New York, USA) is to present the 45-day and one-year follow-up data during a spotlight session for early stage and emerging new technologies in cardiac electrophysiology at this the upcoming AF Symposium (2–4 February, Boston, USA).
“The conformable design of the CLAAS implant enables treatment of a wide range of patients with only two device sizes. This is significant as it streamlines the procedure and reduces imaging requirements,” stated Reddy. “This study demonstrated 100% technical success rate in all 19 patients using a conscious sedation ICE [intracardiac echocardiography]-guided strategy and shows continued clinical success at one year.”
The conscious sedation cases were performed by Reddy and Petr Neuzil (Homolka Hospital, Prague, Czech Republic) as part of the Conformal Prague Study.
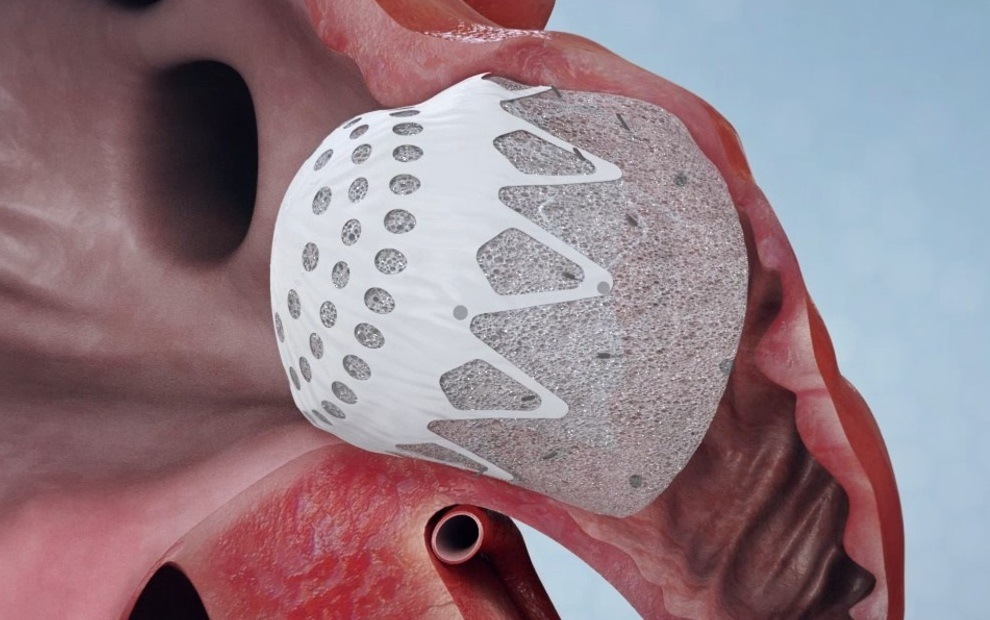
The CLAAS System is designed to seal the LAA in patients with non-valvular atrial fibrillation (AF) to reduce the risk of stroke without the need for anticoagulants. The technology features a proprietary foam-based architecture, the implant addresses a wide spectrum of LAA anatomies with only two sizes.
The system aims to simplify delivery and eliminate the need for procedural transesophageal echocardiogram so that physicians may perform the procedure without general anaesthesia, a significant advancement with the potential to shift clinical practice to a same day, single operator procedure, Confomral Medical said in a press release.
Conformal Medical is actively enrolling patients in the CONFORM pivotal trial, evaluating the safety and efficacy of the CLAAS System compared to other commercially available LAAO devices. The prospective, multicentre, randomised controlled study will enrol approximately 1,600 patients in the USA and Japan and will support US Food and Drug Administration (FDA) premarket approval. Following the randomised phase of the pivotal trial, a prospective single-arm conscious sedation sub-study will be conducted to evaluate the procedural techniques successfully achieved in the Prague study.









