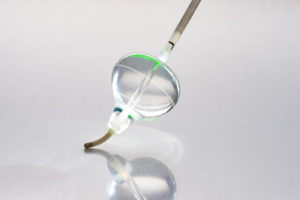
The US Food and Drug Administration (FDA) has granted approval for the HeartLight Excalibur Balloon, a next-generation technology designed for the treatment of paroxysmal atrial fibrillation (PAF).
The Excalibur Balloon leverages the proven universal balloon design of the company’s FDA-approved HeartLight Endoscopic Ablation System and introduces an advanced feature set that optimizes the speed and magnitude of target tissue contact during pulmonary vein isolation (PVI) procedures.
“We have seen encouraging results with the Excalibur Balloon. It obtains an impressive antral position and establishes even more tissue contact, which enables contiguous lesion sets,” said Vivek Y. Reddy, Director of Cardiac Electrophysiology and Helmsley Trust Professor of Medicine at The Icahn School of Medicine at Mount Sinai, New York, USA. “This is a clear advance in the field of balloon-based AF ablation, delivering on the promise of an ultra-compliant balloon.”
In addition to its ultra-compliant design that enables adaptive vein conformance, the Excalibur Balloon also incorporates proprietary Dynamic Response technology. This feature allows the user to make real-time adjustments to balloon size, allowing for optimal tissue contact. These combined features are designed to maximize the engagement of the balloon with the pulmonary
CardioFocus has successfully launched the Excalibur Balloon in Europe following CE Mark approval in September 2017. The technology is now offered at 20 European centers. The company expects to begin a full commercial launch of the Excalibur Balloon in the US in the third quarter of 2018.











