The first commercial implantations of WiSE (wireless stimulation endocardially) technology (EBR Systems) have taken place at James Cook University Hospital, Middlesbrough, UK and Na Homolce Hospital, Prague, Czech Republic.
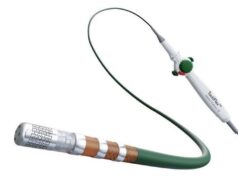
WiSE technology is a wireless endocardial pacing system for cardiac resynchronisation therapy.
WiSE technology is an implantable pacing system capable of delivering energy to the heart without using a pacing lead to conduct the energy. It was designed to address the persistent limitations of current CRT systems; where approximately 30-40% of patients who receive a conventional CRT device fail to respond to therapy or suffer lead-related failures.
Preliminary results from the SELECT-LV study, where patients were implanted with WiSE technology, have demonstrated improvement in 81% of patients who had previously failed conventional CRT treatment.
“What is most promising about the WiSE technology is that we pace from the endocardial, or inside surface of the left ventricle which is a more physiological way to depolarise the myocardium,” says Petr Neuzil, head of Cardiology at Na Homolce Hospital. “This mimics the natural activation of the heart, and may explain why it benefits patients who previously failed treatment.”
“By eliminating the need for a left ventricular lead, we now have the ability to target the exact site where we pace the heart,” says Simon James, consultant cardiologist at James Cook University Hospital. “By selecting a patient-specific pacing site, we expect to be able to increase the number of patients who respond to this therapy.”
WiSE technology has the potential to simplify the whole upgrade process and to reduce complications including infections and lead dislodgements.
All patients implanted with the WiSE technology will be enrolled in the WiCS-LV Post Market Surveillance Registry. This is an international, multicentre registry, designed to study the safety and performance of the technology up to five years post-implant.
Nick Linker, president of British Heart Rhythm Society and chair of the MHRA’s Expert Advisory Group on leadless devices, says, “The Group recognises the importance that all implants are captured within a formal post-approval study during these early years, to help us understand the performance of the technology under real-world conditions of use. We are therefore pleased to see EBR Systems conduct this registry to continue to evaluate the WiSE CRT technology.”