In a new financing round, Kardium has raised an undisclosed amount to fund commercial activities in Europe and Canada, and for clinical testing of the Globe system in the USA. The financing was led by funds and accounts advised by T Rowe Price Associates and included participation from other new and existing Kardium shareholders.
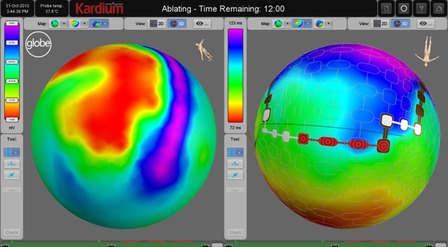
The Globe system is a powerful mapping and ablation solution that consists of a sophisticated catheter with 122 electrodes and advanced software, that together enable rapid pulmonary vein isolation, high resolution mapping, and the ability to ablate anywhere in the atrium.
Having completed successful clinical testing of the Globe system in Switzerland and Germany, Kardium now plans to launch the Globe system with commercial sales in Europe in 2019. Kardium will also start a clinical study in the USA this year.
“This financing round is a reflection of the positive results of our clinical testing of the Globe system in Europe, and the potential of the Globe system to improve the treatment of atrial fibrillation,” said Doug Goertzen, CEO of Kardium. “We are very excited to have T. Rowe Price lead this round of investment. We are looking forward to a long and successful partnership with them and our other investors.”
“Kardium has developed an exciting technology with the Globe system, which we believe has the potential to transform the treatment of atrial fibrillation,” said Ziad Bakri, portfolio manager of T. Rowe Price Health Sciences Fund. “The company has a large addressable market and a strong management team, and we believe that Kardium could become much larger over time.”