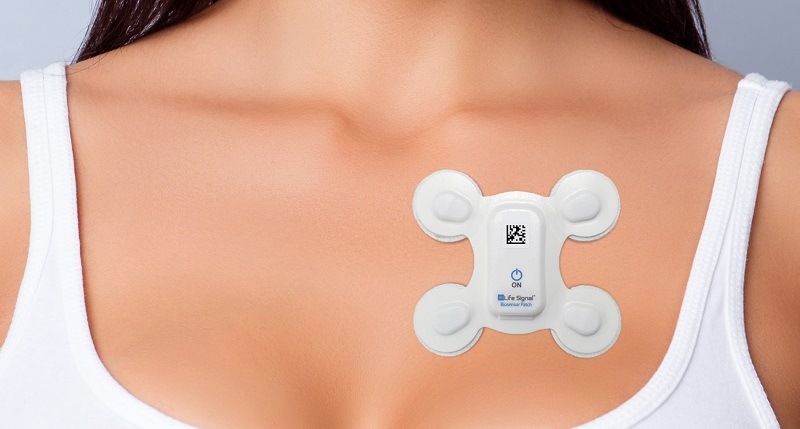
LifeSignals Group has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for the LifeSignals ECG Remote Monitoring Patch platform.
The platform is a wireless remote monitoring system intended for use by healthcare professionals for the continuous collection of electrocardiography (ECG) and heart rate monitoring in ambulatory, hospital, home and healthcare settings. Data is transmitted wirelessly from the LifeSignals wireless medical biosensor LP1250 to a remote secure server for storage and analysis.
Healthcare professionals can remotely access patient data via third-party software for the screening and monitoring of common cardiac arrhythmias such atrial fibrillation, enabling rapid treatment decisions, independent of patient location.
“This FDA clearance represents a major step forward in our drive to untether patient monitoring systems,” said Surendar Magar, co-founder and CEO of LifeSignals. “The interoperable biosensor gives partners access to a ready-to-integrate, multi-parameter medical wearable with a straightforward ecosystem for fast on-boarding. Once in use, it enables remote patient monitoring services to be expanded rapidly and professionals can make faster treatment decisions while patients can be confident of receiving data-driven, personalised therapy”
FDA clearance follows CE marking and HSA approval. The LifeSignals Wireless Medical Biosensor LP1250 will be sold worldwide as a white-labelled device through a network of partnerships from OEMs and telehealth software providers to specialist hospital facilities.













Does the ‘LifeSignals’ ECG Remote Monitoring Patch has the ability to provide a 12-lead ECG signals ?
Regards, Oswald Spiteri [email protected]