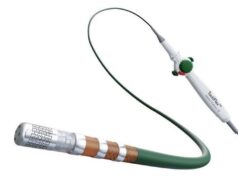
Medtronic has today announced two major milestones relating to the Affera Sphere-360 catheter—the receipt of a CE mark in Europe and completion of the first cases in the Horizon 360 investigational device exemption (IDE) pivotal trial in the USA.
“The Sphere-360 catheter offers an excellent balance between ease of use and consistency in outcomes,” said Tobias Reichlin (Inselspital University Hospital, Bern, Switzerland). “Its design conforms to the pulmonary vein in a unique way that delivers consistent, effective and durable lesions for AF patients, without any rotation of the catheter. With the full integration into the Affera mapping system, it’s an exciting step forward for patient care in Europe.”
According to Medtronic, the Sphere-360 catheter—a “first-of-its-kind”, all-in-one mapping and single-shot pulsed field ablation (PFA) catheter for treating paroxysmal atrial fibrillation (AF)—was designed in response to physician feedback expressing the need for a simple, differentiated workflow and predictable outcomes using a single catheter that accommodates anatomical variations in pulmonary veins. Seamlessly integrated with the Affera mapping and ablation system, the catheter offers an adjustable configuration lattice design that conforms to the shape of the veins and allows energy delivery without the need to rotate the catheter in each position, the company also claims, adding that it has been rapidly adopted by physicians in multiple geographies owing to its excellent safety and durability profile.
One-year results from the Sphere-360 European study were presented at the 2025 Heart Rhythm Society (HRS) annual meeting (24–27 April, San Diego, USA) and subsequently published in the Heart Rhythm Journal. The excellent efficacy, safety and durability demonstrated in this prospective, single-arm, multicentre trial conducted at European centres led to Sphere-360’s recent CE mark.
“Advances in electrophysiology over the past several years are having a significant impact on care for arrhythmia patients, and Affera has been a key part of that impact,” commented Vivek Reddy (Mount Sinai Health System, New York City, USA). “Now, with Sphere-360, we have a new and differentiated single-shot PFA catheter with the potential to deliver the next phase of innovation. It’s an exciting time for physicians and patients, and we’re not slowing down.”
The Horizon 360 IDE study is a prospective, single-arm clinical study taking place at centres across the USA. It is evaluating the safety and effectiveness of the Sphere-360 catheter with the Affera mapping and ablation system in the treatment of paroxysmal AF.
“The achievement of CE mark for Sphere-360, as well as the first cases in the US IDE trial, mark two major achievements in our effort to deliver new and better treatments to AF patients,” said Rebecca Seidel, president of the Cardiac Ablation Solutions business, which is part of the Cardiovascular Portfolio, at Medtronic. “We’re committed to leading in PFA and bringing meaningful innovation at a regular cadence.”