 Medtronic has announced new data from the WRAP-IT study, showing the TYRX absorbable antibacterial envelope (TYRX Envelope) is cost effective for patients at increased risk of infections who receive cardiac implantable electronic devices (CIEDs). The analysis, newly published in Circulation: Arrhythmia and Electrophysiology, demonstrates the envelope’s cost-effectiveness compared to standard-of-care infection prevention strategies in the US healthcare system.
Medtronic has announced new data from the WRAP-IT study, showing the TYRX absorbable antibacterial envelope (TYRX Envelope) is cost effective for patients at increased risk of infections who receive cardiac implantable electronic devices (CIEDs). The analysis, newly published in Circulation: Arrhythmia and Electrophysiology, demonstrates the envelope’s cost-effectiveness compared to standard-of-care infection prevention strategies in the US healthcare system.
This pre-specified analysis of the global WRAP-IT Trial (Worldwide Randomized Antibiotic Envelope Infection Trial) compared costs and patient outcomes between patients who received the TYRX Envelope and patients who received standard-of-care. The TYRX Envelope is cost-effective in the WRAP-IT patient population, with its incremental cost effectiveness ratio (ICER) below the upper willingness to pay threshold of US$150,000, recommended in the American College of Cardiology/American Heart Association practice guidelines on cost/value methodology.
“This analysis builds upon the results of the landmark WRAP-IT study, which showed use of the antibiotic envelope results in a significant reduction in infections, with no increase in complications,” said Bruce Wilkoff, director of Cardiac Pacing and Tachyarrhythmia Devices at Cleveland Clinic (Cleveland, USA), lead author and a paid consultant to Medtronic. “These new data provide evidence that the envelope improves patient outcomes in a cost-efficient way.”
“We are committed to identifying and creating cost-effective innovations like the TYRX Envelope that add value by reducing readmission rates, lowering infection risk, and decreasing hospitalisations,” said Rob Kowal, chief medical officer of the Cardiac Rhythm and Heart Failure division, which is part of the Cardiac and Vascular Group at Medtronic. “During a global pandemic, reducing infections and readmissions is especially important because it means patients have fewer needs for supplemental medical care, which reduces the risk of exposure for themselves and their clinicians.”
Long-term results from WRAP-IT, recently published in Heart Rhythm, show that the effects of TYRX to reduce major CIED infection were sustained through three years of follow-up, which was driven by a significant reduction in pocket infections.
Further, “impact of infection” data from the study, also published in Circulation: Arrhythmia and Electrophysiology, showed that patients who experience a major CIED infection are at a greater than threefold risk of death at one year compared to patients without an infection; experience an additional 9‒18 days in the hospital; suffer impaired quality of life through six months; and pay approximately US$2,000 in out-of-pocket costs to treat the infection.
Based on the WRAP-IT results, an international consensus statement, supported by seven medical societies (including the Heart Rhythm Society) recommends TYRX for the WRAP-IT study population and for patients with high risk factors.
WRAP-IT compared the incidence of major infections in 6,983 patients whose CIED implantation included the TYRX Envelope (3,495) and patients whose procedure did not (3,488), with follow up through 12 months. Primary results published in The New England Journal of Medicine demonstrated the TYRX Envelope reduced the risk of major infection by 40% in patients with CIEDs and reduced pocket infections by 61%, compared to standard of care pre-operative antibiotics. The trial also met its safety objective: the envelope did not increase the risk of complications through 12 months.
The study population included patients receiving a new cardiac resynchronisation therapy defibrillator (CRT-D); and patients receiving a replacement, system revision or generator upgrade of an existing pacemaker, cardiac resynchronisation therapy-pacemaker (CRT-P), implantable cardioverter defibrillator (ICD) or CRT-D. Patients with diabetes, previous history of infection, renal failure, and/or congestive heart failure also are at higher risk for CIED infections.
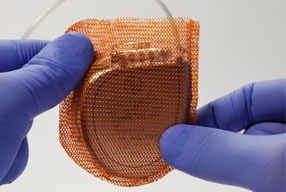
The TYRX Envelope is an absorbable single-use device that holds a cardiac implantable electronic device or implanted neurostimulator. It is designed to stabilise the device after implantation while releasing antimicrobial agents, minocycline and rifampin, over a minimum of seven days. Constructed from a multifilament, knitted absorbable mesh, the envelope is fully absorbed by the body approximately nine weeks after implantation. The device has a one-year shelf life in the USA, Canada, Australia and New Zealand.












