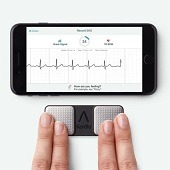
 The KardiaMobile device from AliveCor has received two additional Food and Drug Administration (FDA) 510(k)-cleared indications for arrhythmias that are not atrial fibrillation—for bradycardia, a resting heart rate of between 40–50 beats per minute, and for tachycardia, a heart rate between 100–140 beats per minute. This makes KardiaMobile the only consumer electrocardiogram (ECG) with FDA clearance to detect the three most common arrhythmias of atrial fibrillation, bradycardia, and tachycardia, as well as normal sinus rhythm.
The KardiaMobile device from AliveCor has received two additional Food and Drug Administration (FDA) 510(k)-cleared indications for arrhythmias that are not atrial fibrillation—for bradycardia, a resting heart rate of between 40–50 beats per minute, and for tachycardia, a heart rate between 100–140 beats per minute. This makes KardiaMobile the only consumer electrocardiogram (ECG) with FDA clearance to detect the three most common arrhythmias of atrial fibrillation, bradycardia, and tachycardia, as well as normal sinus rhythm.
Though bradycardia and tachycardia are often benign, they can be indicative of heart disease or other health conditions, such as thyroid disease. A slow or fast heart rate may be asymptomatic or cause symptoms such as dizziness or shortness of breath. In a press release, the company says KardiaMobile users will now be able to detect these arrhythmias and use the insight to inform conversations with their doctor, and that beyond the patient-doctor relationship, KardiaMobile also provides peace of mind by diminishing the number of unclassified readings that users may receive.
AliveCor’s chief executive officer Ira Bahr said in the press release: “No other consumer ECG device in the world can tell you more about your heart than KardiaMobile. Until today, patients have been frustrated when devices label their ECG reading as ‘unclassified’ or ‘inconclusive.’ KardiaMobile is the first personal ECG device that can begin to materially reduce the number of those determinations. Critically, KardiaMobile is also the only personal ECG that can detect atrial fibrillation at heart rates above 120, and heart rates below 40.”












