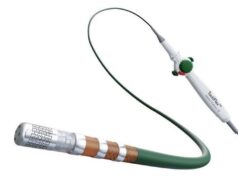
Spectranetics has received US Food and Drug Administration premarket notification 510(k) clearance for the Bridge occlusion balloon for temporary vessel occlusion in cardiac lead extraction procedures. The device builds on the long-standing clinical success and proven procedural safety of cardiac lead extraction. The device is designed to dramatically reduce blood loss in the rare event of a tear, including in the superior vena cava (SVC), providing a “bridge” to surgical intervention.
This clearance will initiate a controlled market release, according to a Spectranetics release, with full market launch at the Heart Rhythm Society’s 37th Annual Scientific Sessions (HRS) in San Francisco, May 4-7, 2016.
Jude Clancy, director of the Lead Management Program at Yale Medical Group, New Haven, USA, and lead physician investigator who will present clinical data on the Bridge occlusion balloon at HRS, says, “I believe Bridge will have a major impact on the field. It is imperative to have the right tools and techniques to support procedural safety. Bridge empowers extractors to take life-saving action while transitioning to surgical repair, driving positive patient outcomes.”
Roger Carrillo, chief of Surgical Electrophysiology, University of Miami Hospital, Miami, USA, says, “With Bridge in place, the surgeon has a clear field of view of the repair site and can quickly take the most appropriate steps for the patient.”