Tag: Boston Scientific
Boston Scientific obtains CE mark to treat right atrial flutter with...
Boston Scientific Corporation has received a CE mark for the Farapoint pulsed field ablation (PFA) catheter, which is intended for use in the treatment...
Boston Scientific gains expanded CE-mark indication for Ingevity+ pacing leads to...
Boston Scientific has received CE-mark approval to expand the indication for its current-generation Ingevity+ pacing leads—thin wires that are implanted in the heart and...
Boston Scientific announces late-breaking data on Farapulse and Watchman FLX technologies...
At the 2025 European Society of Cardiology (ESC) congress (29 August–1 September, Madrid, Spain), Boston Scientific shared two late-breaking presentations pertaining to its Farapulse...
Boston Scientific receives US FDA approval for expanded labelling of Farapulse...
Boston Scientific Corporation has received US Food and Drug Administration (FDA) approval to expand the instructions for use (IFU) labelling for the Farapulse pulsed...
UK NICE recommends pulsed field ablation as AF treatment option
The UK’s National Institute for Health and Care Excellence (NICE) has today published new guidance recommending the use of pulsed field ablation (PFA) in...
Second phase of Boston’s ADVANTAGE AF study meets primary safety and...
Boston Scientific has announced positive 12-month primary endpoint results from the second phase of the ADVANTAGE AF clinical trial evaluating the use of the...
SINGLE SHOT CHAMPION data leave “no doubt” that PFA is non-inferior...
At this year’s European Heart Rhythm Association (EHRA) congress (30 March–1 April 2025, Vienna, Austria), a late-breaking presentation of data from the SINGLE SHOT...
Farapulse PFA system demonstrates superior efficacy to cryoablation catheter in paroxysmal...
New findings from the SINGLE SHOT CHAMPION trial, presented at the ongoing European Heart Rhythm Association (EHRA) congress (30 March–1 April 2025, Vienna, Austria)...
Medtech insight: Pulsed field ablation is a “once-in-a-career opportunity”
Boston Scientific’s vice president (VP) of rhythm management, Caroline Bravo, speaks to Cardiac Rhythm News about the arrival of pulsed field ablation (PFA) for...
Boston Scientific obtains CE mark for next generation of cardiac mapping...
Boston Scientific Corporation has announced it has received CE-mark approval for its navigation-enabled Farawave Nav ablation catheter in the treatment of paroxysmal atrial fibrillation...
Cardiac Rhythm News’ top stories of 2024
From first-time clinical data to major industry debuts, here are Cardiac Rhythm News’ 10 most popular stories of 2024.
1. Abbott joins the pulsed field...
New data presented on Farapulse and Watchman FLX devices at AF...
Boston Scientific announced data supporting the use of the Farapulse pulsed field ablation (PFA) system and the Watchman FLX left atrial appendage closure (LAAC) device,...
US FDA issues alert over potential need for early device replacement...
The US Food and Drug Administration (FDA) has alerted patients, caregivers and healthcare providers about the potential need for early device replacement of Boston...
Boston Scientific announces agreement to acquire Cortex
Boston Scientific Corporation announced today that it has entered into a definitive agreement to acquire Cortex, an Ajax Health company and privately held medical...
Boston Scientific launches next generation of cardiac mapping for Farapulse PFA...
Boston Scientific Corporation has announced that it has received US Food and Drug Administration (FDA) approval of the navigation-enabled Farawave Nav ablation catheter for...
Boston Scientific receives Japanese regulatory approval for Farapulse PFA system
Boston Scientific has announced receipt of Pharmaceuticals and Medical Device Agency (PMDA) approval in Japan for the Farapulse pulsed field ablation (PFA) system. The...
Boston’s Empower leadless pacemaker demonstrates “excellent overall clinical performance” in MODULAR...
Boston Scientific recently announced late-breaking data from the MODULAR ATP clinical trial investigating the pacing performance of the company’s Empower leadless pacemaker. The data...
Boston Scientific appoints Angelo Auricchio as chief medical officer for EMEA...
Boston Scientific has announced the appointment of Angelo Auricchio as chief medical officer (CMO) of the company’s Rhythm Management business in Europe, the Middle...
Advertorial: PFA is now “beyond the phase of excitement”
This advertorial is sponsored by Boston Scientific
The arrival of pulsed-field ablation (PFA) has been heralded by many experts in the field of arrhythmia management...
Medtech insight: Trial pipeline underpins growth strategy for Watchman LAAC device
Investment in a programme of clinical trials will underpin Boston Scientific’s strategy to expand the uptake of its Watchman left atrial appendage closure (LAAC)...
Advertorial: Milestone procedure underscores optimism for future of pulsed field ablation
This advertorial is sponsored by Boston Scientific
Physicians at Na Homolce Hospital (Prague, Czech Republic) have recently completed their 2,000th clinical case using the FARAPULSE...
Healthy or hazardous: Study raises “red flag” on wearable smart device...
A new “red flag-raising” study has provided benchmark data on the safety of smart scales, watches and rings with bioimpedance technology for patients with...
Boston Scientific closes acquisition of Baylis Medical Company
Boston Scientific has announced the close of its acquisition of Baylis Medical Company, a company that offers advanced transseptal access solutions as well as...
Trial underway to evaluate “modular” cardiac rhythm management system
Boston Scientific has announced the start of the MODULAR ATP clinical trial to evaluate the safety, performance and effectiveness of the mCRM modular therapy...
Next-generation Watchman LAAC device gains US FDA approval
Boston Scientific has received US Food and Drug Administration (FDA) approval for the Watchman FLX left atrial appendage closure (LAAC) device. The device is...
Boston Scientific launches its DirectSense technology in the USA
Boston Scientific has announced the US launch of DirectSense technology, a tool for monitoring the effect of radiofrequency (RF) energy delivery during cardiac ablation...
White paper from Heart Rhythm Society suggests Afib treatment has reached...
The Heart Rhythm Society has published a white paper looking at the consequences of oral anticoagulants for managing stroke risk in atrial fibrillation which...
Boston Scientific initiates trial comparing left atrial appendage closure to direct...
According to a press release, Boston Scientific has initiated the OPTION trial to compare safety and effectiveness of the next-generation Watchman FLX left atrial...
UNTOUCHED finds high conversion efficacy and low adverse event rate for...
The Emblem subcutaneous implantable defibrillator system (S-ICD; Boston Scientific) demonstrated a high conversion efficacy and low adverse event rate. Data from the UNTOUCHED trial...
Late-breaking trial highlights positive safety and efficacy data for the LUMINIZE...
Boston Scientific announced data from the AF-FICIENT I study during a late-breaking clinical trial session at the 2019 European Heart Rhythm Association annual congress...
CE mark for Watchman FLX left atrial appendage closure device
Boston Scientific announced it has received CE mark and initiated a limited market release of the next generation Watchman FLX left atrial appendage closure...
Boston Scientific to buy cerebral protection system company
Boston Scientific has signed an agreement to acquire Claret Medical, which developed and commercialised the Sentinel cerebral embolic protection system. The device is used...
Boston Scientific to acquire Cryterion Medical
As part of its plan to expand it atrial fibrillation ablation therapy offering, Boston Scientific Corporation has announced a definitive agreement to acquire Cryterion...
Boston Scientific launches the HeartLogic heart failure diagnostic in Europe
Boston Scientific Europe has announced the launch of the HeartLogic Heart Failure Diagnostic in Europe. With this launch, the first and only diagnostic tool...
Real-world data demonstrates success of SMART Pass on the S-ICD System
Results from an analysis of the LATITUDE database which evaluated the successful reduction of inappropriate shocks using the SMART Pass sensing filter in patients...
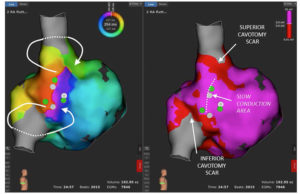
Case report: Usefulness of high resolution mapping with mini-electrodes to...
This article has been sponsored by Boston Scientific.
Case description
A 62-year-old woman, with recent sinus venosus atrial septal defect correction surgery, developed a persistent macroreentrant...
Clinical trial data further demonstrate safety and effectiveness of RHYTHMIA mapping...
Data from the TRUE-HD study has been announced during a late-breaking clinical trial session at the annual congress of the European Heart Rhythm Association...
Short-term DAPT is feasible for patients undergoing percutaneous LAA closure
Felix Weise (Cardioangiologisches Centrum Bethanien, Frankfurt, Germany) and others report in EuroIntervention that a strategy of six weeks’ duration of dual antiplatelet therapy (DAPT)...
TCT 2017: Boston Scientific reveals presentations at conference
Boston Scientific has announced key data will be presented at next week’s Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October–2 November, Denver, USA). For example,...
Boston Scientific announces agreement to acquire Apama Medical
Boston Scientific Corporation has announced a definitive agreement to acquire Apama Medical, a privately-held company that is developing the Apama Radiofrequency (RF) Balloon Catheter...
Boston Scientific gets FDA approval for MRI labelling and launches Resonate...
Boston Scientific has launched the Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) systems featuring the HeartLogic Heart Failure...
Data confirm HeartLogic Diagnostic helps to categorise patients at low or...
Boston Scientific has announced new data from the MultiSENSE (Multisensor chronic evaluation in ambulatory heart failure patients) study, which is evaluating the performance of...
S-ICD technology continues to advance rapidly
The benefits of the Subcutaneous Implantable Cardioverter Defibrillator (S-ICD, Boston Scientific) over transvenous (TV)-ICD, including lower rates of lead-related complications and lower in-hospital complication...
HRS 2017: S-ICDs successful despite a range of comorbidities, and across...
Initial real-world experience with the S-ICD (subcutaneous implantable cardioverter defibrillator) in the USA show that implant success rate is high and short-term complications acceptably...
Boston Scientific receives US FDA approval for Resonate family of high-voltage...
The US Food and Drug Administration (FDA) has approved Boston Scientific’s Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D)...
Boston Scientific announces scheduled presentations at Heart Rhythm Society 2017
Boston Scientific has announced the schedule of key data presentations, including two late-breaking clinical trials that will be featured at the Heart Rhythm Society’s...
UK NICE recommends Boston Scientific CRT-D devices using EnduraLife battery technology
The National Institute for Health and Care Excellence (NICE) has issued medical technology guidance recommending the use of Boston Scientific’s cardiac resynchronisation therapy defibrillators...
Boston Scientific launches Resonate CRT-D platform in Europe
Boston Scientific has launched the Resonate cardiac resynchronisation therapy defibrillator (CRT-D) systems in Europe. This new family of devices features SmartCRT technology, which recently...
Educational supplement: LAA closure for stroke prophylaxis in atrial fibrillation
This educational supplement is only for readers in countries outside France, Japan and the USA
In this educational supplement, sponsored by Boston Scientific, Cardiac Rhythm News explores left...
US FDA approves Boston Scientific Emblem MRI S-ICD system
Boston Scientific has received US Food and Drug Administration (FDA) approval for the Emblem MRI subcutaneous implantable defibrillator (S-ICD) system, as well as magnetic...