 Bhargav Vemulapalli, Shreya Srivastava and John Kassotis (New Brunswick, USA) review the potential use of antibiotic-eluting envelopes (ABEs) based on available clinical data, while underscoring the need for standardised guidelines related to antibiotic prophylaxis.
Bhargav Vemulapalli, Shreya Srivastava and John Kassotis (New Brunswick, USA) review the potential use of antibiotic-eluting envelopes (ABEs) based on available clinical data, while underscoring the need for standardised guidelines related to antibiotic prophylaxis.
Infections associated with cardiac implantable electronic devices (CIEDs) are associated with increased mortality1; posing a substantial financial burden to an overtaxed healthcare system.1–5 Infection rates have increased at rates faster than device implantation1; attributable to increasing numbers of generator replacements and device upgrades in an ageing population with increased comorbidities. CIED infections are serious complications often resulting in device removal with lead extraction. The widespread use of postoperative antibiotics is of questionable efficacy.
The widely accepted prophylaxis for CIEDs is pre-operative antibiotics coupled with meticulous surgical technique. While this practice has reduced the odds of infection by 74%, there remains significant risk in patients only receiving pre-operative antibiotics.4 In fact, the first, double-blinded, randomised control trial designed to determine the efficacy of pre-operative Cefazolin compared with no antibiotic prophylaxis showed that while Cefazolin administration was an independent predictor of infection rates, 15% of participants who had an infection in the follow-up period were on pre-operative Cefazolin.6
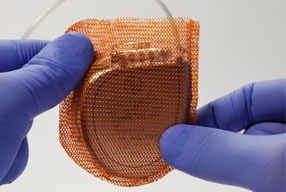
The antibiotic-eluting envelope (ABE) Tyrx (Medtronic), was developed to stabilise the CIED and reduce device infections, cleared for use by the US Food and Drug Administration (FDA) in 2008. This device employs a biocompatible polymer base, designed to break down linearly over time. The polymer acts as a carrier for the antibacterial agents (minocycline and rifampin); agents designed to elute locally, within the surgical pocket, starting within two hours of insertion and continuing for at least seven days following insertion. This device offers coverage against pathogens commonly implicated in CIED infections, including, Coagulase(-)Staphylococcus; Methicillin sensitive and Methicillin resistant Staph Aureus; E coli; H Influenzae; M catarrhalis; and Corynebacterium jeikeium. Despite persisting infection rates and a reflexive increased use of systemic antibiotics, ABE use has yet to achieve universal acceptance. The objective of this review is to frame the potential use of ABE based on available clinical data, while underscoring the need for standardised guidelines related to antibiotic prophylaxis.
Peri-operative pocket irrigation and postoperative antibacterial strategies
A common infection prevention strategy involves administration of pre-operative intravenous antibiotics, peri-operative pocket irrigation, followed by the administration of postoperative oral antibiotics. An international survey revealed 40% and 44% of respondents employed pocket irrigation and postoperative antimicrobial therapy, respectively.8 These practices are used despite results from the PADIT trial which compared a conventional strategy (single-dose of intravenous (IV) cefazolin or IV vancomycin (penicillin allergic patients), administered within 60 minutes of skin incision compared to an incremental treatment strategy (combining both pre-operative IV cefazolin and IV vancomycin, pocket irrigation prior to incision closure and two days of postoperative oral antibiotics. This study concluded there was no benefit to extended antibiotic use.9
Efficacy, safety, and economic cost of antibiotic-eluting envelopes
WRAP-IT, a multicentre, randomised controlled clinical trial, found that ABE use resulted in a 40% reduction in major infections and a 61% reduction in pocket infections with no significant difference in complications or increased procedural time.10 Further analysis showed that the effect of ABEs is sustained up to 36 months after implementation.11 Additionally, Tyrx demonstrated efficacy against Staph-related pocket infections.11
In a meta-analysis, investigators showed that ABE therapy with standard infection prophylaxis resulted in a reduction in all infections following CIED insertion.12 While ABE use has been shown to reduce CIED infections, clinical findings suggested an incremental benefit in those patients at higher risk for infection13 and in more complex CIEDs (e.g. cardiac resynchronization therapy [CRT] defibrillators and implantable cardioverter defibrillators [ICDs]).14 Even still, as per the European Heart Rhythm Association (EHRA) guidelines, the Tyrx ABE is the only form of localised delivery of antibiotics supported by consensus.15
In conjunction with its clinical benefits, Tyrx remains cost-effective.16 A US economic analysis demonstrated that ABE is cost-effective for patients at increased risk of infections compared to standard-of-care infection prevention strategies. The cost-effectiveness ratio associated with ABE therapy was well below the upper willingness to pay threshold of US$150,000, the recommended threshold of the American College of Cardiology/American Heart Association practice guidelines on cost/value methodology.17 This suggests that the improved outcomes secondary to ABE therapy add value to the healthcare system.
Summary
CIED infections are serious complications associated with significant morbidity and mortality. The most common prophylactic strategies have failed to affect CIED infection rates. Recent clinical evidence has shown significant reductions in CIED infection rates with adjunctive ABE use, with either patients deemed at high risk or higher risk device implantations. In the authors’ opinion, it is imperative that implanters factor ABE use as a treatment strategy for all patients undergoing device implantation.
Conclusion
ABE use has emerged as a well-established, clinically validated, and economically viable treatment option effective in the reduction of CIED related infections. The authors want to highlight the EHRA International Consensus Statement that supports the use of the Tyrx ABE and underscore the need for operators to consider use of ABE as part of an antibiotic prophylactic strategy.
About the authors

John Kassotis is a Professor of Medicine serving as director of Cardiovascular Fellowship and director, Cardiovascular Education, at Rutgers Robert Wood Johnson Medical School, New Brunswick, USA. A Professor whose prior training includes both Clinical Cardiac Electrophysiology and Advanced Heart Failure.
Shreya Srivastava recently received her MPH from the Johns Hopkins Bloomberg School of Public Health and is now a fourth-year medical student at Rutgers Robert Wood Johnson Medical School. Shreya will begin her residency in internal medicine in July. She has a particular interest in cardiology and would like to pursue a career in academia.
Barrgav Vemulapalli is a second-year medical student and a the Chancellor’s Global Scholar at the Rutgers Robert Wood Johnson Medical School. Bhargav has a particular interest in global health and the environment, Bhargav aspires to pursue a career as an cardiologist.
References
1 Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001-1006. doi:10.1016/j.jacc.2011.04.033
2 Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and Cost Associated With Cardiovascular Implantable Electronic Device Infections. Arch Intern Med. 2011;171(20):1821-1828. doi:10.1001/archinternmed.2011.441
3 Baddour LM, Epstein AE, Erickson CC, et al. A Summary of the Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. J Am Dent Assoc. 2011;142(2):159-165. doi:10.14219/jada.archive.2011.0058
4 de Bie MK, van Rees JB, Thijssen J, et al. Cardiac device infections are associated with a significant mortality risk. Heart Rhythm. 2012;9(4):494-498. doi:10.1016/j.hrthm.2011.10.034
5 Wilkoff BL, Boriani G, Mittal S, et al. Impact of Cardiac Implantable Electronic Device Infection: A Clinical and Economic Analysis of the WRAP-IT Trial. Circ Arrhythm Electrophysiol. 2020;13(5):e008280. doi:10.1161/CIRCEP.119.008280
6 Oliveira JC de, Martinelli M, Nishioka SAD, et al. Efficacy of Antibiotic Prophylaxis Before the Implantation of Pacemakers and Cardioverter-Defibrillators. Circ Arrhythmia Electrophysiol. 2009;2(1):29-34. doi:10.1161/CIRCEP.108.795906
7 Costa A Da, Kirkorian G, Cucherat M, et al. Antibiotic Prophylaxis for Permanent Pacemaker Implantation. Circulation. 1998;97(18):1796-1801. doi:10.1161/01.CIR.97.18.1796
8 Traykov V, Bongiorni MG, Boriani G, et al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: results of a worldwide survey under the auspices of the European Heart Rhythm Association. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2019;21(8):1270-1279. doi:10.1093/europace/euz137
9 Krahn AD, Longtin Y, Philippon F, et al. Prevention of Arrhythmia Device Infection Trial: The PADIT Trial. J Am Coll Cardiol. 2018;72(24):3098-3109. doi:10.1016/j.jacc.2018.09.068
10 Tarakji KG, Mittal S, Kennergren C, et al. Antibacterial Envelope to Prevent Cardiac Implantable Device Infection. N Engl J Med. 2019;380(20):1895-1905. doi:10.1056/NEJMoa1901111
11 MR S, GR C, BL W, et al. Clinical Presentation, Timing, and Microbiology of CIED Infections: An Analysis of the WRAP-IT Trial. JACC Clin Electrophysiol. 2021;7(1):50-61. doi:10.1016/J.JACEP.2020.07.021
12 Kumar A, Doshi R, Shariff M. Role of antibiotic envelopes in preventing cardiac implantable electronic device infection: A meta-analysis of 14 859 procedures. J Arrhythmia. 2020;36(1):176-179. doi:10.1002/joa3.12262
13 Asbeutah AAA, Salem MH, Asbeutah SA, Abu-Assi MA. The role of an antibiotic envelope in the prevention of major cardiac implantable electronic device infections: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99(26):e20834. doi:10.1097/MD.0000000000020834
14 Pranata R, Tondas AE, Vania R, Yuniadi Y. Antibiotic envelope is associated with reduction in cardiac implantable electronic devices infections especially for high-power device-Systematic review and meta-analysis. J Arrhythmia. 2020;36(1):166-173. doi:10.1002/joa3.12270
15 Blomström-Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Associatio. EP Eur. 2020;22(4):515-549. doi:10.1093/EUROPACE/EUZ246
16 Wilkoff BL, Boriani G, Mittal S, et al. Cost-Effectiveness of an Antibacterial Envelope for Cardiac Implantable Electronic Device Infection Prevention in the US Healthcare System From the WRAP-IT Trial. Circ Arrhythmia Electrophysiol. 2020;13:1073-1082. doi:10.1161/CIRCEP.120.008503
17 Anderson JL, et al. ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures. Circulation. 2014;129:2329-2345.













I am. happy to know that. Electrophysioogists are now implanting pacemakers and other devices using the Tyrx antibiotic envelope. My electrophysiologist i Dr, Sangha ( Dartmouth Hitchcock. Lebanon NH Implanted my Azure pacemaker in February 2018. He was aware of the. Hospital acquired staph infections. And wanted to prevent infection
It gives the patient great confidence that they will not get an infection and also helps stabilize the device. Thank you for the article Dr. Vemulllapalli