Tag: CRT
Leadless pacing shows high rate of success at six months
Almost 70% of patients treated with a leadless left ventricular (LV) endocardial pacing system had a favourable clinical response at six months, a post-market...
Cardiac magnetic resonance safe and effective at measuring the contraction response...
Researchers have used cardiac magnetic resonance (CMR) imaging for the first time to demonstrate the mechanistic improvements in cardiac contractility consequent to cardiac resynchronisation...
FDA grants Breakthrough Device Designation for the WiSE CRT
EBR Systems has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its WiSE cardiac resynchronisation therapy (CRT)...
CRT patients undergoing lead extraction are at no higher risk than...
Transvenous lead extraction in cardiac resynchronisation therapy (CRT) patients is not associated with increased 30-day mortality vs. non-CRT patients. The study found that age,...
New analyses show that AdaptivCRT algorithm reduces AF episodes in patients
Two real-world analyses featuring the AdaptivCRT algorithm reinforce that its use is linked to a reduction in atrial fibrillation (AF) episodes, as well as...
US$50 million raised for SOLVE-CRT study on wireless CRT technology
EBR Systems has raised US$50 million to conduct the global SOLVE-CRT (stimulation of the left ventricle endocardially) study. SOLVE-CRT is a pivotal clinical trial intended to establish safety...
Boston Scientific gets FDA approval for MRI labelling and launches Resonate...
Boston Scientific has launched the Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) systems featuring the HeartLogic Heart Failure...
Cardiac resynchronisation therapy guided by imaging: The next step for optimal...
Jonathan Behar (London, UK) co-inventor for the Guide CRT platform—shortlisted as a 2017 finalist for the EHRA Inventors Award—details the benefits of this novel...
EHRA Inventors Award ideas focus on cardiac remote monitoring and CRT...
For the second-year running, the European Heart Rhythm Association (EHRA) held the EHRA Inventors Award competition at EHRA-EUROPACE CARDIOSTIM (18‒21 June, Vienna, Austria), a...
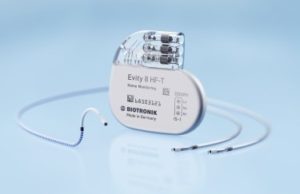
Biotronik’s Evity quadripolar CRT-P released in Japan
Biotronik’s Evity cardiac resynchronisation therapy pacemaker (CRT-P) has been launched in Japan. It is the company’s highest performing CRT-P, with a battery life of...
CRT optimisation: Why a sensor-based strategy may be the optimal solution
This video has been sponsored by LivaNova.
Over the past two decades there have been several optimisation strategies, whether they are echo-guided or EKG-guided, aimed...
Cardiac resynchronisation therapy upgrade is less favourable than de novo implantations
Results from a large multicentre observational study have shown that patients undergoing cardiac resynchronisation therapy (CRT) upgrade had worse clinical response and long-term survival...
Insights into current heart failure management
Piotr Ponikowski (Centre for Heart Disease, Clinical Military Hospital, Wroclaw, Poland), chairperson of the 2016 European Society of Cardiology (ESC) Guidelines for the diagnosis...
Quadra Allure MP CRT-P gets CE mark approval for MRI labeling
St Jude Medical has announced CE mark approval for magnetic resonance (MR) conditional labeling for the company’s Quadra Allure MP cardiac resynchronisation therapy pacemaker (CRT-P).
The...
Medtronic gets FDA approval for MRI compatible cardiac rhythm and heart...
Medtronic has announced US Food and Drug Administration (FDA) approval for its suite of cardiac rhythm and heart failure devices and leads to be...
ESC 2016: Remote monitoring for heart failure may not improve clinical...
Two randomised clinical trials presented at a Hot Line session of the European Society of Cardiology Congress (ESC; 27‒31 August, Rome, Italy) have shown...
EBR Systems receives FDA approval to begin IDE study of its...
EBR Systems has announced the US Food and Drug Administration (FDA) has granted an Investigational Device Exemption (IDE) for its WiSE (Wireless Stimulation Endocardially) technology for cardiac...
Cardiac resynchronisation therapy with SonR automated feature helps to reduce hospitalisation...
Results from the RESPOND-CRT trial have shown a 35% risk reduction in hospitalisation at two years for heart failure patients implanted with cardiac resynchronisation...
Wireless CRT has the potential to improve response in heart failure...
The world’s only wireless endocardial pacing system for cardiac resynchronisation therapy (CRT)—WiSE (Wireless Stimulation of the Endocardium) CRT System (EBR Systems)—is showing promise treating...