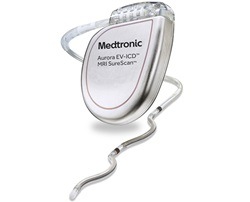
Medtronic has received US Food and Drug Administration (FDA) approval for the Aurora EV-ICD MRI SureScan (extravascular implantable cardioverter-defibrillator) and Epsila EV MRI SureScan defibrillation lead to treat dangerously fast heart rhythms that can lead to sudden cardiac arrest (SCA).
The Aurora EV-ICD system is a first-of-its-kind device that provides the benefits of traditional, transvenous ICDs with a lead placed under the breastbone, outside of the heart and veins. The Aurora EV-ICD delivers defibrillation, anti-tachycardia pacing (ATP), and back-up (pause-prevention) pacing therapies via a device similar in size, shape, and longevity to traditional, transvenous ICDs.
FDA approval of this system includes its proprietary procedure implant tools, and was supported by global pivotal trial results showing the system’s safety and effectiveness that were published in The New England Journal of Medicine.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” said Bradley P Knight, (Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, USA) a co-author of the study. “Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology.”
Results from the study show that the device’s effectiveness in delivering defibrillation therapy at implant was 98.7%, and there we no major intraprocedural complications, nor any unique complications observed related to the EV ICD procedure or system compared to transvenous and subcutaneous ICDs. Additionally, 33 defibrillation shocks were avoided by having ATP—which paces the heart to interrupt and terminate a dangerous rhythm—programmed “on.” And at six months, 92.6% of patients (Kaplan-Meier estimate) were free from major system and/or procedure-related major complications such as hospitalisation, system revision, or death.
Medtronic will obtain real-world performance and safety data on the Aurora system in the Enlighten global post-approval registry, a prospective, non-randomised, observational, multicentre study, expected to last five years and enrol approximately 1,000 patients. First implants in Enlighten and first commercial implants worldwide were recently conducted by Lucas VA Boersma, (St Antonius Hospital, Nieuwegein, The Netherlands), and a limited launch is underway in select European countries.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, and who have not had a prior sternotomy and do not need chronic bradycardia (abnormally slow heartbeat) pacing.











